Crystallographers and materials scientists use the density of a metallic sample to infer its likely crystal structure.
Question:
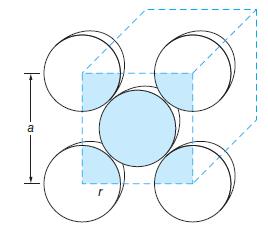
Crystallographers and materials scientists use the density of a metallic sample to infer its likely crystal structure. The density of copper (Cu) is 8.96 g/cm3 and its atomic radius is 0.128 nm. Is the copper crystal more likely to be face-centered cubic or body-centered cubic? (See Figure 10-57.)
Figure 10-57
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





