Question: Note in Table 9-2 that the equilibrium separation of the KBr and RbCl molecules is very nearly equal. Compute the exclusion-principle repulsion for these molecules.
Note in Table 9-2 that the equilibrium separation of the KBr and RbCl molecules is very nearly equal. Compute the exclusion-principle repulsion for these molecules.
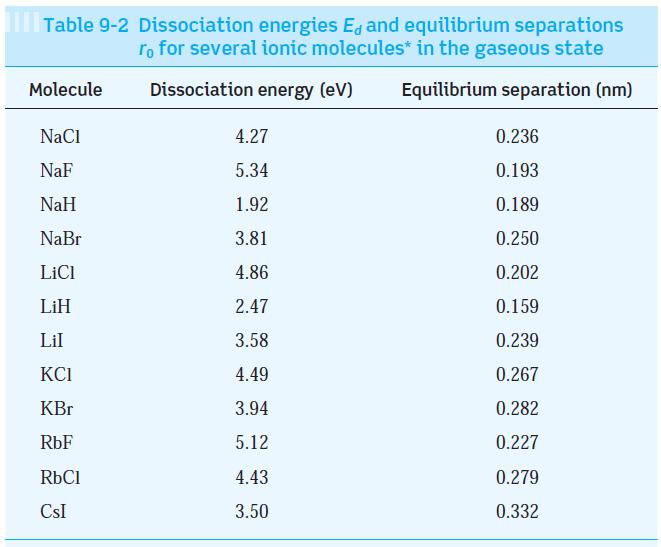
Table 9-2 Dissociation energies E, and equilibrium separations ro for several ionic molecules* in the gaseous state Dissociation energy (eV) Equilibrium separation (nm) Molecule NaCl NaF NaH NaBr LiCl LiH Lil KCI KBr RbF RbCl CSI 4.27 5.34 1.92 3.81 4.86 2.47 3.58 4.49 3.94 5.12 4.43 3.50 0.236 0.193 0.189 0.250 0.202 0.159 0.239 0.267 0.282 0.227 0.279 0.332
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
For KBr Uc 1440eVnm 0282nm E 394... View full answer

Get step-by-step solutions from verified subject matter experts


