Three isotopes of hydrogen occur in nature; ordinary hydrogen, deuterium, and tritium. Their nuclei consist of, respectively,
Question:
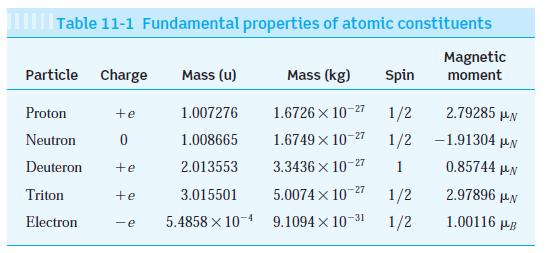
Three isotopes of hydrogen occur in nature; ordinary hydrogen, deuterium, and tritium. Their nuclei consist of, respectively, 1 proton, 1 proton and 1 neutron (deuteron), and 1 proton and 2 neutrons (triton). The masses of the three nuclei are given in Table 11-1.
(a) Use Equation 4-26 to determine Rydberg constants for deuterium and tritium.
(b) Determine the wavelength difference between the Balmer a lines of deuterium and tritium.
(c) Determine the wavelength difference between the Balmer a lines of hydrogen and tritium.
Table 11-1
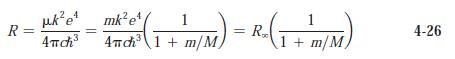
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





