The affinity of integrins for matrix components can be modulated by changes to their cytoplasmic domains: a
Question:
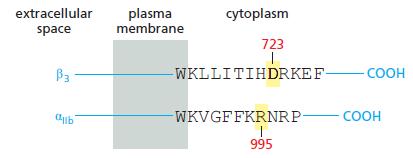
The affinity of integrins for matrix components can be modulated by changes to their cytoplasmic domains: a process known as inside-out signaling. You have identified a key region in the cytoplasmic domains of αIIbβ3 integrin that seems to be required for inside-out signaling (Figure Q19–3). Substitution of alanine for either D723 in the β chain or R995 in the α chain leads to a high level of spontaneous activation, under conditions where the wild-type chains are inactive. Your advisor suggests that you convert the aspartate in the β chain to an arginine (D723R) and the arginine in the α chain to an aspartate (R995D). You compare all three α chains (R995, R995A, and R995D) against all three β chains (D723, D723A, and D723R). You find that all pairs have a high level of spontaneous activation, except D723 vs R995 (the wild type) and D723R vs R995D, which have low levels. Based on these results, how do you think the αIIbβ3 integrin is held in its inactive state?
Figure Q19-3
Step by Step Answer:

Molecular Biology Of The Cell
ISBN: 9780815344322
6th Edition
Authors: Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter





