The movements of single motor-protein molecules can be analyzed directly. Using polarized laser light, it is possible
Question:
The movements of single motor-protein molecules can be analyzed directly. Using polarized laser light, it is possible to create interference patterns that exert a centrally directed force, ranging from zero at the center to a few piconewtons at the periphery (about 200 nm from the center). Individual molecules that enter the interference pattern are rapidly pushed to the center, allowing them to be captured and moved at the experimenter’s discretion.
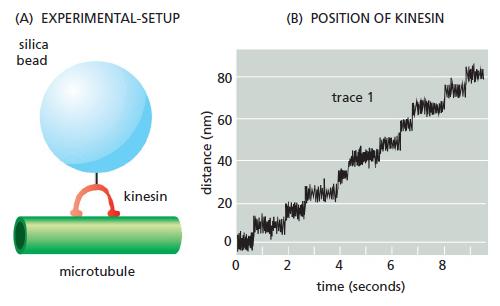
Using such “optical tweezers,” single kinesin molecules can be positioned on a microtubule that is fixed to a coverslip. Although a single kinesin molecule cannot be seen optically, it can be tagged with a silica bead and tracked indirectly by following the bead (Figure Q16–3A).
In the absence of ATP, the kinesin molecule remains at the center of the interference pattern, but with ATP it moves toward the plus end of the microtubule. As kinesin moves along the microtubule, it encounters the force of the interference pattern, which simulates the load kinesin carries during its actual function in the cell. Moreover, the pressure against the silica bead counters the effects of Brownian (thermal) motion, so that the position of the bead more accurately reflects the position of the kinesin molecule on the microtubule.
A trace of the movements of a kinesin molecule along a microtubule is shown in Figure Q16–3B. A. As shown in Figure Q16–3B, all movement of kinesin is in one direction (toward the plus end of the microtubule). What supplies the free energy needed to ensure a unidirectional movement along the microtubule?
B. What is the average rate of movement of kinesin along the microtubule?
C. What is the length of each step that a kinesin takes as it moves along a microtubule?
D. From other studies it is known that kinesin has two globular domains that can each bind to β-tubulin, and that kinesin moves along a single protofilament in a microtubule. In each protofilament, the β-tubulin subunit repeats at 8-nm intervals. Given the step length and the interval between β-tubulin subunits, how do you suppose a kinesin molecule moves along a microtubule?
E. Is there anything in the data in Figure Q16–3B that tells you how many ATP molecules are hydrolyzed per step?
Figure Q16-3
Step by Step Answer:

Molecular Biology Of The Cell
ISBN: 9780815344322
6th Edition
Authors: Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter





