A certain natural terpene produced peaks in its mass spectrum at m/z 204, 111, and 93 (among
Question:
A certain natural terpene produced peaks in its mass spectrum at m/z 204, 111, and 93 (among others). On the basis of this and the following information, elucidate the structure of this terpene. Justify each of your conclusions.
(a) Reaction of the unknown terpene with hydrogen in the presence of platinum under pressure results in a compound with the molecular formula C15H30.
(b) Reaction of the terpene with ozone followed by dimethyl sulfide produces the following mixture of compounds (1 mol of each for each mole of the unknown terpene):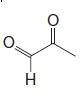
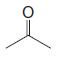
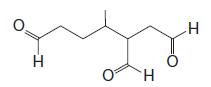
(c) After writing the structure of the unknown terpene, circle each of the isoprene units in this compound. To what class of terpenes does this compound belong (based on the number of carbons it contains)?
Step by Step Answer:

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder





