Reserpine is a natural product belonging to the family of alkaloids (see Special Topic H in WileyPLUS
Question:
Reserpine is a natural product belonging to the family of alkaloids (see Special Topic H in WileyPLUS ). Reserpine was isolated from the Indian snakeroot Rauwolfia serpentina. Clinical applications of reserpine include treatment of hypertension and nervous and mental disorders. The synthesis of reserpine, which contains six chirality centers, was a landmark accomplishment reported by R. B. Woodward in 1955. Incorporated in the synthesis are several reactions involving amines and related nitrogen-containing functional groups, as we shall see on the following page.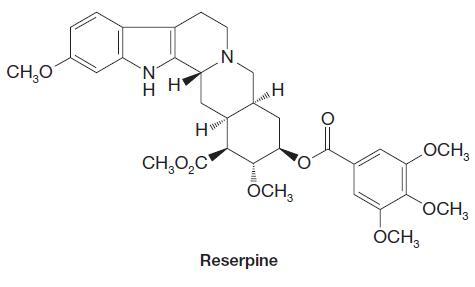
(a) The goal of the first two steps shown in the scheme on the following page, prior to the 1formation of the amide, is the preparation of a secondary amine. Draw the structure of the products labeled A and B from the first and second reactions, respectively. Write a mechanism for the formation of A.
(b) The next sequence of reactions involves the formation of a tertiary amine together with closure of a new ring. Write curved arrows to show how the amide functional group reacts with phosphorus oxychloride (POCl3) to place the leaving group on the bracketed intermediate.
(c) The ring closure from the bracketed intermediate involves a type of electrophilic aromatic substitution reaction characteristic of indole rings. Identify the part of the structure that contains the indole ring. Write mechanism arrows to show how the nitrogen in the indole ring, via conjugation, can cause electrons from the adjacent carbon to attack an electrophile. In this case, the attack by the indole ring in the bracketed intermediate is an addition–elimination reaction, somewhat like reactions that occur at carbonyls bearing leaving groups.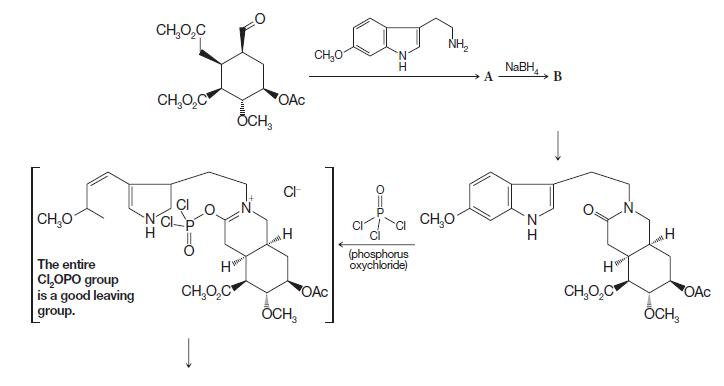
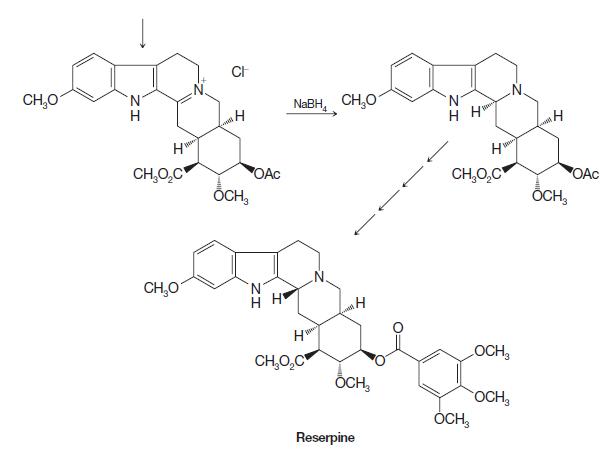
Step by Step Answer:

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder





