The 1H NMR signals for the aromatic hydrogens of methyl p-hydroxybenzoate appear as two doublets at approximately
Question:
The 1H NMR signals for the aromatic hydrogens of methyl p-hydroxybenzoate appear as two doublets at approximately 7.05 and 8.04 ppm (δ). Assign these two doublets to the respective hydrogens that produce each signal. Justify your assignments using arguments of relative electron density based on contributing resonance structures.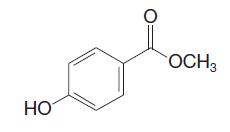
Transcribed Image Text:
HO 。 OCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
My argument on my assignment is as follows i The chemical shift of the downfield ...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
Order the 1H NMR signals of the following compounds by chemical-shift position (lowest to highest). Which one is the most upheld? The most downfield? (a) H3C-CH3 (b) H2C==CH2 (c) H3C-O-CH3 (d) (e)...
-
The 1H NMR signal for bromoform (CHBr3) appears at 2065 Hz when recorded on a 300-MHz NMR spectrometer. (a) What is the chemical shift of this proton? (b) Is the proton in CHBr3 more shielded or less...
-
The 1H NMR signal for bromoform (CHBr3) appears at 2065 Hz when recorded on a 300-MHz NMR spectrometer. (a) What is the chemical shift of this proton? (b) Is the proton in CHBr3 more shielded or less...
-
Compare and contrast megaloblastic anemia caused by vitamin B12 deficiency and that caused by folic acid deficiency. (10)
-
Let M be the median (in points) and R be the range (in points) of four test scores (all in points), x1, x2, x3, and x4, where the scores are listed from smallest to largest. a. Write a formula for...
-
Did Qwest admit to improper accounting before or after the SEC suit?
-
The distribution of the ages of the winners of the Tour de France from 1903 to 2016 is approximately bell-shaped. The mean age is 27.9 years, with a standard deviation of 3.3 years. Use the...
-
Like the hole in a bagel, any hole in Finagle A Bagels information and accounting systems means less dough for the company. Copresidents Alan Litchman and Laura Trust and their management team could...
-
Identify the reasons for disclosure below as they are (OR) are not for public interest or benefit. Options 1. Not for public interest or benefit 2. Not for public interest or benefit 3. For public...
-
Regal Entertainment Group operates the largest chain of movie theaters in the U.S. Classify each of the following items found in the company's financial statements included in the Form 10-K for the...
-
The structure of thyroxine, a thyroid hormone that helps to regulate metabolic rate, was determined in part by comparison with a synthetic compound believed to have the same structure as natural...
-
The following reaction sequence was used by E. J. Corey (J. Am. Chem. Soc. 1969, 91, 56755677) at the beginning of a synthesis of prostaglandin F 2 and prostaglandin E 2 . Explain what is involved in...
-
Calculate y'. y = (1 - x 2 + ) -1
-
Diamond and Turf Inc. is considering an investment in one of two machines. The sewing machine will increase productivity from sewing 160 baseballs per hour to sewing 288 per hour. The contribution...
-
10-Convert the following C code to MIPS. Assume the address of base array is associated with $s0, n is associated with $s1, position is associated with $t0, c is associated with $t1, d is associated...
-
Explain the term R, Value and what are the factors effect on R, value?
-
This problem is a small experiment. The goal of this problem is to give students an opportunity to experimentally observe whether an insertion sort would be faster than a quicksort when an input size...
-
1. Recall the matrix A, in the finite-difference solution of the boundary value problem discussed CO Th, p(BCS) in class (steady state heat conduction). It was stated in class that p(B) 1+sin Th cos...
-
Let A be an n ( n matrix with integer entries. Prove that A is nonsingular and A-1 has integer entries if and only if det(A) = (1.
-
Charles owns an office building and land that are used in his trade or business. The office building and land were acquired in 1978 for $800,000 and $100,000, respectively. During the current year,...
-
Explain whether these elimination reaction would be a good way to prepare thesealkenes: Cl H,O CH,OH + KOH CI EIOH PHCH=CHCH, b) PHCH CHCH; + NaOEt
-
Explain which of these reactions would provide a better synthesis of2-pentene: Br CH,OH CH,CH,CHCH,CH, + CH;0 CH,CH=CHCH,CH3 Br CH, CH CH=CHCH CH, CH,CHCH,CH,CH; + CH,0
-
Show the products of thesereactions: Br CH,OH CH3CH,CHCH,CH, + CH,0 CH,CH-CHCH,CH3 Br CH;OH CH,CHCH,CH,CH; + CH,0 CH.CH-CHCH,CH; CH, CH-CH2 2 b) ELOH Br Br
-
In January 2023, Marty's Fine Pens, a business carried on as a sole proprietorship, sells a limited-edition fountain pen for $125,000. The cost of the pen is $63,000. There is a down payment of...
-
An 90000 loan is amortized by payments of $1850 at the end of every 6 months at a rate of 2% compounded monthly 1. Construct a partial amortization schedule showing the last 2 payments 2. determine...
-
Kelso's has a return on equity of 15.2 percent, a debt-equity ratio of 44 percent, a capital intensity ratio of 1.08, a current ratio of 1.25, and current assets of $138,000. What is the profit...

Study smarter with the SolutionInn App


