(a) Assuming the presence of acids (AH) and bases (A) as needed, give a curved-arrow mechanism for...
Question:
(a) Assuming the presence of acids (AH) and bases (A¯) as needed, give a curved-arrow mechanism for the isomerization of isopentenyl pyrophosphate into dimethylallyl pyrophosphate.
(b) In some organisms, geranyl pyrophosphate is converted by hydrolysis into one of two enantiomeric tertiary alcohols: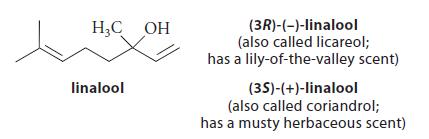
Assuming the presence of acids (AH), bases (A¯), and water as needed, give a curved-arrow mechanism for this transformation. (Ignore stereochemistry.)
(c) Each of the stereoisomeric linalools in part (b) is converted into an epoxide A by CyP450, and the epoxide spontaneously forms the following compound B. Neglecting stereochemistry, suggest a structure for A; and, assuming the presence of H3O+ and H2O, provide a curved-arrow mechanism for the conversion of A into B.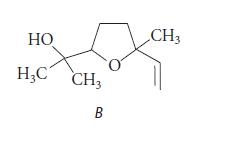
Step by Step Answer:






