(a) Explain why 2,4-pentanedione (Eq. 22.15) contains much less enol form in water (15%) than it does...
Question:
(a) Explain why 2,4-pentanedione (Eq. 22.15) contains much less enol form in water (15%) than it does in hexane (92%).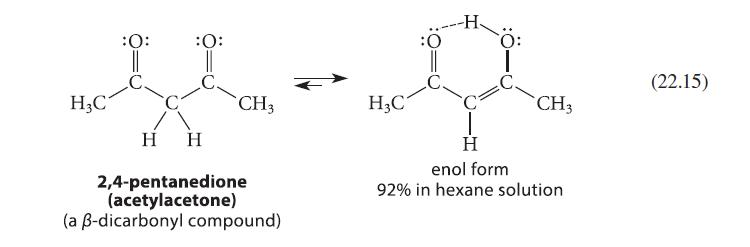
(b) Explain why the same compound has a strong UV absorption in hexane solvent (λmax = 272 nm, ϵ = 12,000), but a weaker absorption in water (λmax = 274 nm, ϵ = 2050).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





