Question: (a) Explain why compound A, when treated with one equivalent of NaOEt, followed by acidification, is completely converted into compound B. (b) Write a curved-arrow
(a) Explain why compound A, when treated with one equivalent of NaOEt, followed by acidification, is completely converted into compound B.
(b) Write a curved-arrow mechanism for this conversion.
(c) Give the structure of the only product formed when diethyl a-methyladipate (compound C) reacts in the Dieckmann condensation. Explain your reasoning.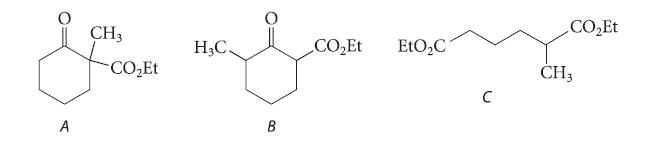
A CH3 -COEt H3C. B COEt EtO2C .COzEt CH3
Step by Step Solution
3.25 Rating (154 Votes )
There are 3 Steps involved in it
a b An ordinary Claisen condensation is driven to completion by ionization of ... View full answer

Get step-by-step solutions from verified subject matter experts


