(a) The dipole moments of pyrrole and pyrrolidine are similar in magnitude but have opposite directions. Explain,...
Question:
(a) The dipole moments of pyrrole and pyrrolidine are similar in magnitude but have opposite directions. Explain, indicating the direction of the dipole moment in each compound.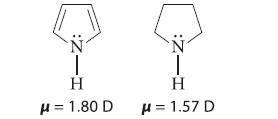
(b) Explain why the dipole moments of furan and pyrrole have opposite directions.
(c) Should the dipole moment of 3,4-dichloropyrrole be greater than or less than that of pyrrole? Explain.
Transcribed Image Text:
-Z: H H = 1.80 D -Z: T H H = 1.57 D
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Because nitrogen is an electronegative atom the CN bond dipoles in pyrrolidine are directed towards the nitrogen and their resultant is also directe...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The dipole moments of pyrrole and pynolidine are similar in magnitude but have opposite directions. Explain, indicating the direction of the dipole moment in each compound. Should the dipole moment...
-
The compounds FCl and ICl have dipole moments that are similar in magnitude (0.9 and 0.7 D, respectively) but opposite in direction. In one compound, chlorine is the positive end of the dipole; in...
-
The dipole moments of furan and tetrahydrofuran are in the same direction. One compound has a dipole moment of 0.70 D, and the other has a dipole moment of 1.73 D. Which is which?
-
A close company which prepares accounts to 31 March each year is owned and managed by a single shareholder/director who is not a Scottish taxpayer and who is paid a salary of 5,000 per month. In...
-
(a) Use the result of part (e) of Problem 72 (Equation 40-23) to show that after N head-on collisions of a neutron with carbon nuclei at rest, the energy of the neutron is approximately (0.714)NE0,...
-
Botany Bay, Inc., a maker of casual clothing, is considering four projects. Because of past financial difficulties, the company has a high cost of capital at 15%. Which of these projects would be...
-
Select a publicly traded company or use a company assigned by your instructor. Using the firms most recent Form 10-K report (accessed through the SEC EDGAR database or from the companys website),...
-
Green Lighting Supply plans inventory levels (at cost) at the end of each month as follows: May, $271,000; June, $226,000; July, $209,000; and August, $241,000. Sales are expected to be June,...
-
The accounting records of NuTronics, Incorporated, include the following information for the year ended December 31: Inventory of materials Inventory of work in process Inventory of finished goods...
-
Explain the position of substitution observed in the bromination of thiophene-3-carboxylic acid shown in Eq. 26.18. Draw the arenium ion intermediates for all possible positions of substitution and...
-
Imidazole is a base; the pK a of its conjugate acid is 6.95. On which nitrogen does imidazole protonate?
-
The figure shows the population density (people per square mile) for the 50 states in the United States, based on an estimate from the U.S. Census Bureau. The regions are the Midwest (MW), Northeast...
-
Mr. Smart Lal commenced his trading business in the name of Exotica Trading Company on 1st April 2017 with a capital of 1,000,000 and a loan from the State Bank of India amounting to 500,000. At...
-
Show a pipeline execution diagram for the third iteration of this loop, from the cycle in which we fetch the first instruction of that iteration up to (but not including) the cycle in which we can...
-
Based upon the given Trial Balance and the additional information, prepare the statement of profit and loss of Shivam Industries Limited for the year ended 31st March 2017: Trial Balance of Shivam...
-
Identify the parent(s), subsidiaries and associates, if any, in the following structure: B 50% 60% A C D 60% 40%
-
Explain why power in itself a sufficient basis is not to determine control.
-
For a random sample of 36 items and a sample mean of 211, compute a 95% confidence interval for if the population standard deviation is 23.
-
Calculate the change in entropy when 100 kJ of energy is transferred reversibly and isothermally as heat to a large block of copper at (i) 0 C, (ii) 50 C.
-
Propose reasonable fragmentation mechanisms that explain why The EI mass spectrum of benzoic acid shows major peaks at m/z = 105 and m/z = 77.
-
Give the structure for an isomer of compound A that has a melting point of 208C and NMR spectra that are almost identical to those of A.
-
Provide a structure for each of the following compounds. C 9 H 10 O 3 : IR 2400-3200, 1700, 1630 cm 1 NMR: 1.53 (3H, t, J = 8 Hz); 4.32 (2H, q, J = 8 Hz); 7.08, 8.13 (4H, pair of leaning...
-
4. What is the time complexity of fun ( ): int fun(int n) { int count = 0%;B for (int i = n; i > 0; i /= 2) = for (int j count += 1; 0; j < i; j++) } return count;
-
Please may I ask for advice on how to approach this question for a constitutional law: To what extent is the concept of separation of powers an effective mechanism of ensuring constitutionalism?
-
3. What is the time complexity of this algorithm? while (low list [mid] ) low = mid + 1; else break; }

Study smarter with the SolutionInn App


