An optically active compound A (C 9 H 11 Br) reacts with sodium ethoxide in ethanol to
Question:
An optically active compound A (C9H11Br) reacts with sodium ethoxide in ethanol to give an optically inactive hydrocarbon B (NMR spectrum in Fig. P16.58). Compound B undergoes hydrogenation over a Pd/C catalyst at room temperature to give a compound C, which has the formula C9H12. Give the structures of A, B, and C.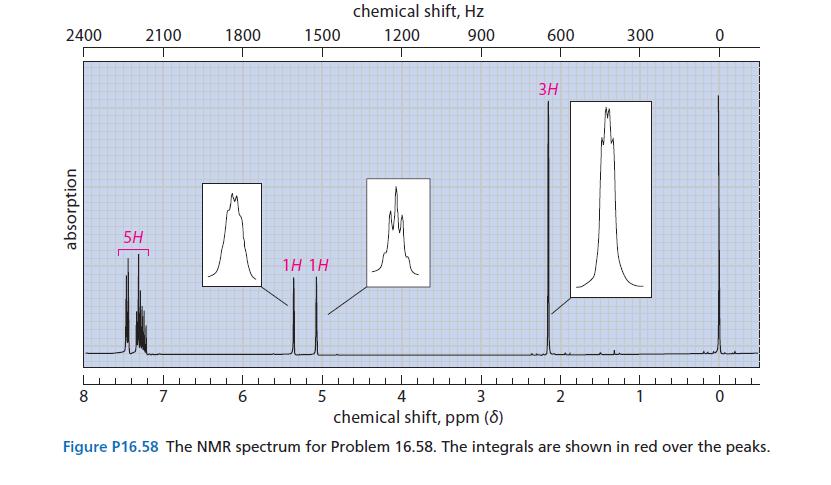
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





