Anticipating the isolation of the potentially aromatic hydrocarbon B, a group of chemists irradiated compound A with
Question:
Anticipating the isolation of the potentially aromatic hydrocarbon B, a group of chemists irradiated compound A with ultraviolet light. Compound C was obtained as a product instead of B.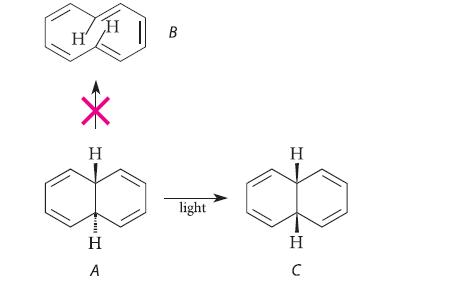
(a) Explain why compound B might be expected to be unstable in spite of its cyclic array of 4n + 2 π electrons.
(b) Explain why the formation of compound B is allowed by the pericyclic selection rules.
(c) Account for the formation of the observed product C.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





