From the specific rotations shown in Eq. 24.18, calculate the percentages of - and -D- glucopyranose present
Question:
From the specific rotations shown in Eq. 24.18, calculate the percentages of α- and β-D- glucopyranose present at equilibrium.Compare your answer to the data given in Table 24.1.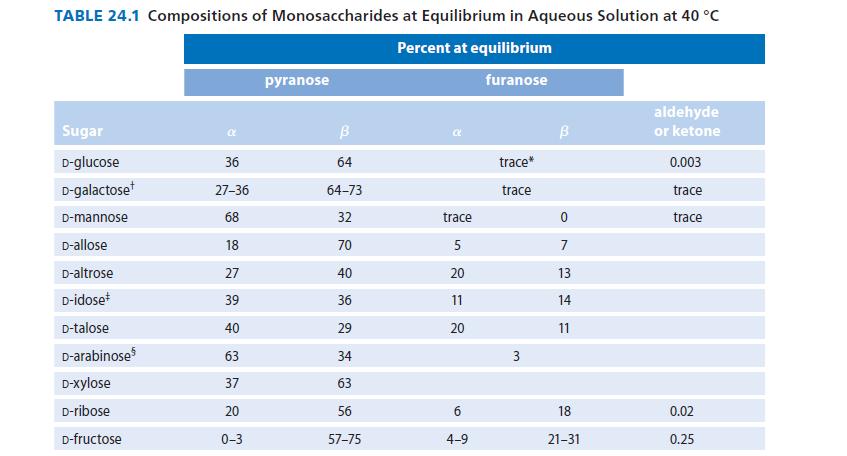
![HO HOCH HO OH -H OH a-anomer [a] =+112 degrees ml g-1 dm- acid or base H20 HOCH HO OH equilibrium mixture:](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/9/3/2/84265716f2a8015e1701932840806.jpg)
Transcribed Image Text:
TABLE 24.1 Compositions of Monosaccharides at Equilibrium in Aqueous Solution at 40 °C Percent at equilibrium furanose Sugar D-glucose D-galactose D-mannose D-allose D-altrose D-idose D-talose D-arabinose D-Xylose D-ribose D-fructose 36 27-36 68 18 27 39 40 63 37 20 0-3 pyranose B 64 64-73 32 70 40 36 29 34 63 56 57-75 trace 5 20 11 20 6 4-9 trace* trace 3 В 0 7 13 14 11 18 21-31 aldehyde or ketone 0.003 trace trace 0.02 0.25
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Each form of the sugar contributes its own optical rotation in proportion to the amoun...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1. What are the real and anticipated arguments that could be made by those at Harrison Industries who may try to convince Donna to go along with the accounting for future severance payments? Include...
-
Calculate the percentages of -D-glucose and -D-glucose present at equilibrium from the specific rotations of -D-glucose, -D-glucose, and the equilibrium mixture. Compare your values with those given...
-
The two most common isotopes of uranium are 235U and 238U. (a) Compare the number of protons, the number of electrons, and the number of neutrons in atoms of these two isotopes. (b) Using the...
-
Hansel Electronics has the following: If Hansel has 7,000 units on hand at December 31, the cost of ending inventory under the average-cost method is: (a) $84,000. (b) $70,000. (c) $56,000. (d)...
-
The Lorentz transformation for y and z is the same as the classical result: y = y and z = z. Yet the relativistic velocity transformation does not give the classical result uy = uy and uz = uz....
-
Use the following ratio information for Johnson International and the industry averages for Johnson's line of business to: a. Construct the DuPont system of analysis for both Johnson and the...
-
Fat cells in humans are composed almost entirely of pure triglycerides with an average density of about 900 kg/m 3 . If 20% of the mass of a 70 kg students body is fat (a typical value), what is the...
-
Jefferson Jerome is interested in purchasing "Art Specialists Inc.", an auction house. The company receives the right to sell art but not to purchase the art themselves for a 5% commission. Art...
-
2. Write a JavaScript and add a div element in the body. Add the following style to the div element: background-color: #D94A38 . width:90px . height:20px padding:40px First when a mouse-button is...
-
Using the curved-arrow notation, fill in the details for base-catalyzed mutarotation of glucopyranose. Begin by removing a proton from the hydroxy group at carbon-1.
-
Using the curved-arrow notation, fill in the details for acid-catalyzed mutarotation of glucopyranose shown in Eq. 24.19. Begin by protonating the ring oxygen. HO HO HOCH2 a-anomer OH HO opening of...
-
1. What do you think of the way in which Ali was approached by Dr. Shields about her violation of the dress code? Does this seem advisable to you? 2. How much of a role do you think different...
-
How do changes in the real interest rate affect the IBL and current and future consumption?
-
What is the logic behind the intertemporal budget constraint? On what assumptions is it based, and how is its slope interpreted?
-
On what assumptions did Keynes base his theory of consumption? How does his theory relate to intertemporal choice?
-
Suppose a firm has a great idea: overnight shipping. This idea will decrease costs for many businesses and will therefore result in a more efficient economy. If the entrepreneurs who create this...
-
What arguments should be considered in assessing the burden that government debt imposes on future generations?
-
The Zimmerman Agency conducted a study for Residence Inn by Marriott of business travelers who take trips of five nights or more. According to this study, 37% of these travelers enjoy sightseeing...
-
Controls can be identified based on their function. The functions are preventive, detective, and corrective. A. True B. False
-
For each compound, state whether its bonding is covalent, ionic, or a mixture of covalent and ionic. (a) NaCl (b) NaOH (c) CH3Li (d) CH2CI2 (e) NaOCH3 (f) HCO2Na (g) CF4
-
(a) Both PCI3 and PCI5 are stable compounds. Draw Lewis structures for these two compounds. (b) NCI3 is a known compound, but all attempts to synthesize NCI5 have failed. Draw Lewis structures for...
-
Draw a Lewis structure for each species. (a) N2H4 (b) N2H2 (c) (CH3)2NH2CI (d) CH3CN (e) CH3CHO (f) CH3S(O)CH3 (g) H2SO4 (h) CH3NCO (i) CH3OSO2OCH3 (j) CH3C(NH)CH3 (k) (CH3)3CNO
-
How does the implementation of Kaizen philosophy contribute to the perpetuation of continuous improvement within complex organizational structures?
-
discuss the argument and criticism in Jean-Jacques Rousseau in the origin and the foundation of the inequality among mankind in relation to Peter Hogg's Democracy, law and inequality in...
-
For a certain multi-state lottery the prize was $12,200,000 that was paid out over 20 years with each year payments of $610,000.00 paid at the end of each year. If money is worth 6.4%, compounded...

Study smarter with the SolutionInn App


