How many moles of permanganate are required to oxidize one mole of toluene to benzoic acid? (Use
Question:
How many moles of permanganate are required to oxidize one mole of toluene to benzoic acid? (Use H2O and protons to balance the equation.)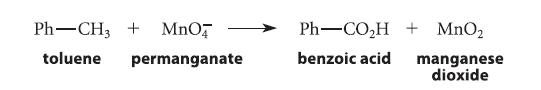
Transcribed Image Text:
Ph-CH3 + MnO toluene permanganate Ph-CO₂H + MnO₂ benzoic acid manganese dioxide
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The glib way to work this problem is to balance the changes in oxidation states ...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How many moles of dichromate are required to oxidize one mole of ethanol to acetaldehyde? ethanol dichromate acetaldehyde
-
Tranquil Teas makes bottled iced teas and uses a process cost system. Each day, Tranquil produces 250 bottles of tea; each period, Tranquil produces 23,000 bottles; each year, Tranquil produces...
-
Use the solution of Problem 19-12 to calculate how many moles of photons of UV light ( = 220 nm) would be required to drive the four-electron oxidation of H2O by NADP+ under standard conditions to...
-
Selected answer is incorrect During substantive procedures, performing analytical procedures satisfies which primary audit objective: Cutoff Accuracy Existence Completeness 2 answers
-
Refer to the data in PE 6-18. Assume that during the following year, 20 customers bring in 80 tires for warranty repairs. The labor and supplies associated with these repairs were $900 and $350,...
-
Explain why, for large corporations, a low pay performance relationship is to be expected.
-
Although the customer loyalty project at Petrie Electronics had gone slowly at first, the past few weeks had been fast-paced and busy, Jim Watanabe, the project manager, thought to himself. He had...
-
Recording Seven Typical Adjusting Entries Dittmans Variety Store is completing the accounting process for the year just ended, December 31, 2011. The transactions during 2011 have been journalized...
-
Research Web-based database technologies and identify a database management system (other than SQL Server, MySQL, or Oracle) that is used to deploy applications to the Web and the cloud. Discuss the...
-
Write a curved-arrow mechanism for the following oxidation of 2-heptanol, which proceeds in 82% yield. OH 2-heptanol + Ph3C+ BFT a relatively stable carbocation 2-heptanone + HBF4 + Ph3CH
-
Contrast the products expected when 2-methyl-3-pentanol is treated with (a) HBr/H 2 SO 4 or (b) Ph 3 PBr 2 . Explain.
-
In what ways is PHP similar to JavaScript?
-
Fama and French (2020) proposed two additional models that allow for timevarying risk parameters. Write the equations for these two conditional models. In empirical tests, which model was the best...
-
Use the Internet to research the database integration features of an ERP software package and a CRM software package. The number of pages will be indicated by your instructor. a. Learn about the ERP...
-
Fama and French (2015) tested their five-factor model in the sample period 19632013. What test asset portfolios did they use? Was the five-factor model supported?
-
Given their new geometry of the parabola, how did Kolari, Liu, and Huang (KLH) (2021) define the expected returns for the special case of orthogonal portfolios I and ZI on the parabola? Describe...
-
Manufacturing Co. has been negotiating with Imran Elina regarding the sale of some property that represented an old manufacturing site which is now surplus to requirements. Because part of the site...
-
After Maria and Tatsuo are divorced, their 2 minor children continue to live with Maria. Pursuant to their divorce decree, Tatsuo pays Maria $1,000 per month in child support and $1,800 per month in...
-
Graph the following conic sections, labeling vertices, foci, directrices, and asymptotes (if they exist). Give the eccentricity of the curve. Use a graphing utility to check your work. 10 5 + 2 cos 0
-
What general kind of reaction does NAD+ carry out?
-
What general kind of reaction does FAD carry out?
-
Why arent the glycolysis and gluconeogenesis pathways the exact reverse of one another?
-
Beta of a portfolio. The beta of four stocks-G, H, I, and J-are 0.43, 0.78, 1.24, and 1.58, respectively. What is the beta of a portfolio with the following weights in each asset: ? What is the beta...
-
Given: 400 lb force along the cable DA. Find: The force FDA in the Cartesian vector form. Plan: 1. Find the position vector 2. Obtain the force vector as and the unit vector "DA. FDA = 400 lb up .
-
A savings plan makes annual deposits of $4,170 each at the end of the first 2 years, followed by 6 end-of-the-year deposits of $4,920 each. The annual rate of return is 6.60%. What is the amount that...

Study smarter with the SolutionInn App


