In each of the following parts, explain why the first compound has a higher boiling point than
Question:
In each of the following parts, explain why the first compound has a higher boiling point than the second, despite a lower molecular mass.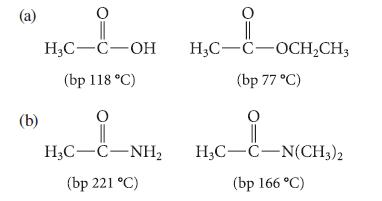
Transcribed Image Text:
(a) (b) H₂C-C-OH (bp 118 °C) O H3C-C-NH₂ (bp 221 °C) H₂C-C-OCH₂CH3 (bp 77 °C) O H3C-C-N(CH3)2 (bp 166 °C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a Explanation Acetic acid CH3COOH has higher boiling point 118 C than ethyl acetate CH3COOCH2CH3 bp ...View the full answer

Answered By

Vijesh J
My passion to become a tutor is a lifetime milestone. Being a finance and marketing professional with hands-on experience in wealth management, portfolio management, team handling and actively contributing in promoting the company. Highly talented in managing and educating students in most attractive ways were students get involved. I will always give perfection to my works. Time is the most important for the works and I provide every answer on time without a delay. I will proofread each and every work and will deliver a with more perfection.
4.70+
5+ Reviews
15+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In each of the following parts. Explain why the first compound has a higher boiling pc-rint than the second, despite a lower molecular mass. (bp 221 C) (bp 166 C)
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Explain why a. H2O has a higher boiling point than CH3OH(65oC). b. H2O has a higher boiling point than NH3(- 33oC). c. H2O has a higher boiling point than HF (20C).
-
Do you agree or not? Specialization of labor and better use of capital goods can initially generate increasing marginal output (returns) for a firm in the production of a good.
-
Was the Calcu-Folio properly classified as a briefcase or a binder?
-
Marjorie is an accountant and Alana is an attorney. They have been business acquaintances for about 10 years. They meet every Friday at 6 P.M. at a local tavern to socialize. As always happens with...
-
Assume the same information as in question 4. Also assume that beginning work in process had \($6,000\) in conversion cost and that \($84,000\) in conversion is added during this period. What is the...
-
The accounting records of Clear Photography, Inc., reflected the following balances as of January 1, 2012: Cash .......... $18,000 Beginning inventory .... 13,500 (150 units @ $90) Common stock...
-
A pre-anoxic MLE activated sludge process has the following influent wastewater characteristics and is operated with an anoxic volume of 500 m3 and MLVSS of 2000 mg/L at 20 oC. Given that influent...
-
Without consulting tables, arrange the compounds within each of the following sets in order of increasing boiling point, and give your reasoning. (a) 1-hexanol, 2-pentanol, tert-butyl alcohol (b)...
-
For the following problems, see Table 8.2 for structures and dipole moments. Explain your reasoning in each case. (a) With which one of the following solvents is DMSO not miscible: water, acetone,...
-
Why is customer-profitability analysis a vitally important topic to managers?
-
By defining development to include more than just growth in per capita income, it is implicitly assumed that growth in per capita income alone is not sufficient to ensure poverty reduction and...
-
American and Mexican workers can each produce 10 T-shirts per day.An American worker can produce 18 dozen brownies per day, while a Mexican worker can produce 10 dozen brownies per day.To simplify...
-
Pat operated a mail order business during the year. Revenues from the business were $34,000 and the cost of merchandise purchased was 18,000 of which $4,000 was still held as inventory at December...
-
What are the different formats available for presenting the income statement? What elements does it show that are important for decision making?
-
What did Reagan mean when he said: As long as the [Brandenburg] Gate is closed, as long as this scar of a wall is allowed to stand, it is not only the German question that remains open, but the...
-
Have you ever worked for the minimum wage? If so, for how long? Would you favor increasing the minimum wage by a dollar? By two dollars? By five dollars? Explain your reasoning.
-
Listed below are several terms and phrases associated with basic assumptions, broad accounting principles, and constraints. Pair each item from List A (by letter) with the item from List B that is...
-
Name these compounds. Discuss.
-
Explain which compound has the higher melting point.
-
Explain which compound has the higher boiling point. Discuss in detail.
-
In the movie, The Matrix Reloaded, Neo (80 kg) jumps towards Agent Smith (600 kg) who jumps towards Neo. When they collide, they grab hold of one another. If Neo has a velocity of 25.0 m/s to the...
-
A ski jumper (m = 70 kg) has a speed of 40.0 m/s at the bottom of a ramp. She has a speed of 39.4 m/s when she leaves contact with the top of the ramp. The ramp is 8 m long and tipped at an angle of...
-
A 615 kg car traveling at 54 m/s can brake and comes to a stop in 47 m. What is the force applied by the brakes in Newtons?

Study smarter with the SolutionInn App


