Tert-butyl chloride undergoes solvolysis in either acetic acid or formic acid. Both solvents are protic, donor solvents,
Question:
Tert-butyl chloride undergoes solvolysis in either acetic acid or formic acid.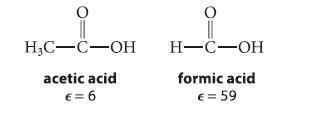
Both solvents are protic, donor solvents, but they differ substantially in their dielectric constants ϵ.
(a) What is the SN1 solvolysis product in each solvent?
(b) In one solvent, the SN1 reaction is 5000 times faster than it is in the other. In which solvent is the reaction more rapid, and why?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





