The allyl cation can be represented by the following resonance structures. (a) What is the bond order
Question:
The allyl cation can be represented by the following resonance structures.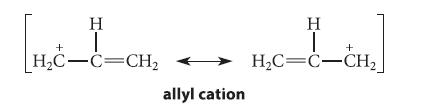
(a) What is the bond order of each carbon–carbon bond in the allyl cation?
(b) How much positive charge resides on each carbon of the allyl cation?
(c) Although the preceding structures are reasonable descriptions of the allyl cation, the following cation cannot be described by analogous resonance structures.
Explain why the structure on the right is not a reasonable resonance structure.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





