The UV spectrum of p-nitrophenol in aqueous solution is shown in Fig. P18.59 (spectrum A). When a
Question:
The UV spectrum of p-nitrophenol in aqueous solution is shown in Fig. P18.59 (spectrum A). When a few drops of concentrated NaOH are added, the solution turns yellow and the spectrum changes (spectrum B). On addition of a few drops of concentrated acid, the color disappears and spectrum A is restored. Explain these observations.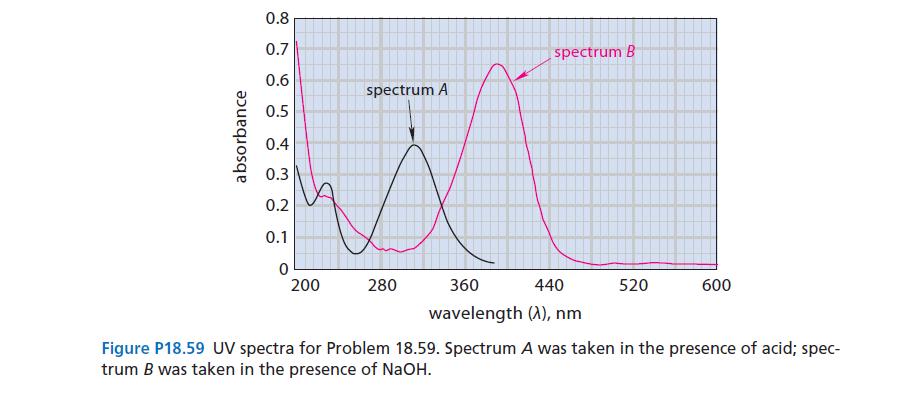
Transcribed Image Text:
absorbance 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 200 spectrum A 280 spectrum B 360 440 wavelength (A), nm 520 600 Figure P18.59 UV spectra for Problem 18.59. Spectrum A was taken in the presence of acid; spec- trum B was taken in the presence of NaOH.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Because NaOH can ionize a phenol particularly a relatively aci...View the full answer

Answered By

William Otieno
I am a professional tutor and a writer with excellent skills that are important in serving the bloggers and other specialties that requires a great writer. The important aspects of being the best are that I have served so many clients with excellence
With excellent skills, I have acquired very many recommendations which have made it possible for me to survive as an excellent and cherished writer. Being an excellent content writer am also a reputable IT writer with essential skills that can make one turn papers into excellent result.
4.70+
83+ Reviews
354+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A student was studying terpene synthesis, and she wanted to make the compound shown here. First she converted 3-bromo-6-methylcyclohexene to alcohol A. She heated alcohol A with sulfuric acid and...
-
The UV spectrum of 1-phenylprop-2-en-1-ol shows an intense absorption at 220 nm and a weaker absorption at 258 nm. When this compound is treated with dilute sulfuric acid, it rearranges to an isomer...
-
The UV spectrum of an unknown compound shows values of λmax at 225 nm (ε = 10,000) and at 318 nm 1e = 402. The mass spectrum shows a molecular ion at m/z 96 and a prominent...
-
1. Enis falsely accuses Monalisa of stealing from Island Tours, Inc., their employer. Enis's statement is defamatory only if a. a third party hears it. b. Monalisa has not been caught. c. the...
-
Roy Akins was the accounting manager at Zelco, Inc., a tire manufacturer, and he played golf with Hugh Stallings, the CEO, who was something of a celebrity in the community. The CEO stood to earn a...
-
A random sample of 100 observations results in 40 successes. a. What is the point estimate for the population proportion of successes? b. Construct the 90% confidence interval for the population...
-
Consider the following cash flow profile and assume MARR is 10 percent/year. a. What does Descartes' rule of signs tell us about the IRR(s) of this project? b. What does Norstrom's criterion tell us...
-
The air pollution project discussed in the chapter has progressed over the past several weeks, and it is now the end of week 8. Lester Harky would like to know the value of the work completed the...
-
Tl-208 decays through a beta emission and has a half-life of 3.05 minutes. Besides the beta emission, there are several gamma rays associated with the decay and these gamma emissions occur at...
-
Phenols, like alcohols, are Brnsted bases. (a) Write the reaction in which the oxygen of phenol reacts as a base with the acid H 2 SO 4 . (b) On the basis of resonance and polar effects, decide...
-
Contrast the reactivities of cyclohexanol and phenol with each of the following reagents, and explain. (a) Aqueous NaOH solution (b) NaH in THF (c) Triflic anhydride in pyridine, 0 8C (d)...
-
Many state and federal laws place possible future liabilities on companies as a result of environmental pollution, gender discrimination, safety requirements, and other social responsibilities. In...
-
How social problems of vulnerable populations have perpetuated or are affected by poverty on a micro and macro level.
-
Exar Document Specialist is a service firm specialising in document preparation. Their office consists of 14 units with 5 employees to each unit. Each document specialist is equipped with a word...
-
What are some biases, stereotypes, preconceptions and or assumptions about clients which you specifically need to be aware of? what are some areas for improvement? What do you intend on doing to...
-
Using the above video, why are the perspectives on the interview so different, and were they fair? Explain. Why would or wouldn't someone offer Felix the position? Explain. What are the 2 insights...
-
Define both of concepts active and passive audiences. no copy and paste from the website, I need original sentences of the definition.
-
Design Arts Associates is an interior decorating firm in Berlin. The following costs were incurred in the firms contract to redecorate the mayors offices. Direct material used...
-
Government is advised to tax goods whose demand curves are inelastic if the goal is to raise tax revenues. If the goal is to discourage consumption, then it ought to tax goods whose demand curves are...
-
A commercial synthesis of folic acid consists of heating the following three compounds with aqueous sodium bicarbonate. Propose reasonable mechanisms for the reactions that lead to folic acid. Hint:...
-
Outline a preparation of benzylamine using the Gabriel synthesis.
-
Give structures for compounds R-W: CHal H.O) Ag20 H20 N-Methylpiperidine- R (CH16NI) 0-5 (C,HyNO) at CHal H2O
-
8. State the condition for the equation Ax = b to have a unique solution, where A is a square matrix. Show that the system of linear equations x + ky + (k 1)z = 4 (k+1)x + (3k+1)x + (k-2)y + (k + 2)z...
-
1. (20pts) Consider the following state-space system, x = [12] x + [1] u u, y = [0_1]X 3 a) (5pts) Derive the controllability and observability matrices and verify that the system is both observable...
-
4. A particle of mass m moves vertically under gravity in a medium which offers a resistance proportional to the square of the speed. The terminal speed of fall is U. The particle is projected...

Study smarter with the SolutionInn App


