Consider the structure of nitrosobenzene: (a) Draw the resonance structures of the sigma complex formed when nitrosobenzene
Question:
Consider the structure of nitrosobenzene:
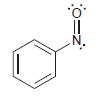
(a) Draw the resonance structures of the sigma complex formed when nitrosobenzene reacts with an electrophile (E+) at the ortho position.
(b) Draw the resonance structures of the sigma complex formed when nitrosobenzene reacts with an electrophile (E+) at the meta position.
(c) Draw the resonance structures of the sigma complex formed when nitrosobenzene reacts with an electrophile (E+) at the para position.
(d) Compare the stability of the intermediates from parts a€“c of this problem, and then predict whether the nitroso group is ortho-para directing or meta directing.
(e) The nitroso group has a lone pair adjacent to the ring (suggesting that it could be an activator), yet it also has a π bond to a heteroatom in conjugation with the ring (suggesting that it could be a deactivator). Experiments reveal that this group is a deactivator. Based on this information, identify which of the following groups is expected to have properties most similar to the nitroso group, and explain your choice:
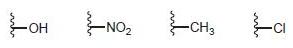
Step by Step Answer:






