Consider the structures of cis-decalin and trans-decalin: (a) Which of these compounds would you expect to be
Question:
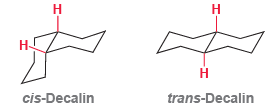
(a) Which of these compounds would you expect to be more stable?
(b) One of these two compounds is incapable of ring flipping. Identify it and explain your choice.
Transcribed Image Text:
Н cis-Decalin trans-Decalin
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
a CisDecalin has three gauche interactions while transdecalin has o...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of these compounds would you expect to be more soluble in water? Explain? CHCHCHCHCOH or CH3CHCHCHCHCOH
-
Consider the structures of cis-1,2-dimethylcyclopropane and trans-1,2-dimethylcyclopropane: (a) Which compound would you expect to be more stable? Explain your choice. (b) Predict the difference in...
-
Which of these compounds would you expect to have the highest boiling point? Explain. [Section 24.4] CH3CH CH CH OH CHC=CH HCOCH
-
On 28 April 2020, Mr Guna, CEO of Econ Engineering Malaysia, proposed to complete an abandoned boiler project that no one had dared to revive. He knew that the project was 60% complete before it was...
-
The Bretton Woods institutions were created in the aftermath of World War II. Many believe they are obsolete or are in need of massive redesign. The way forward must reference the road that has been...
-
Which of the items in the following list are liabilities and which of them are assets? (a) Loan to A. Sangster (b) We are owed by a customer (c) Equipment (d) Bank overdraft (e) Inventory of goods...
-
Describe the common models of managed care organizations.
-
What SQL command(s) would you use to add the date on which an employee was hired to the EMPLOYEE table represented in Figure? Name this new attribute Employment_ Date. Assume that the employees were...
-
dy 1. Find and simplify. dx tanx (a) y= (3 marks) (b) y x cosh (In x) (3 marks) (c) + sinh 2y = y - cosh 2x (4 marks)
-
Munson Performance Auto, Inc., modifies 375 autos per year. The manager, Adam Munson, is interested in obtaining a measure of overall performance. He has asked you to provide him with a multifactor...
-
A housing bubble occurs when _________ drive(s) prices more than fundamental factors. a. The price of gasoline b. A homes expected future price c. Interest rate changes d. Property tax increases
-
Atorvastatin is sold under the trade name Lipitor and is used for lowering cholesterol. Annual global sales of this compound exceed $13 billion. Assign a configuration to each chirality center in...
-
What are the six main cultural dimensions analyzed in the Hofstede framework?
-
How to calculate the Pearson's r correlation between peer interaction and the cognitive skills development for 22 students, if their scores in cognitive as (Abby, 22), (Babs, 16), (Fred, 30), and...
-
How has the real estate industry evolved or changed throughout time? Write a critically discussion.
-
Current price of the company 12%, semiannual, noncallable bonds with 15 years remaining to maturity is $ 1,153.72. There are 70,000 bonds. what is the market interest rate of the debt?
-
How much will I need to deposit into my account at the beginning of each month for the next 50 years if I want $2,700,000 at the end, assuming my account makes 6% pa compounded quarterly over that...
-
How can a SWOT analysis be useful in finance career? Critically discuss step by step process of SWOT analysis. Explain briefly
-
A steak is more expensive at Dinos Steak House than at Ricks Prime Rib House. Therefore, the quality of a steak at Dinos Steak House is better than the quality of a steak at Ricks Prime Rib House....
-
The Zwatch Company manufactures trendy, high-quality moderately priced watches. As Zwatch's senior financial analyst, you are asked to recommend a method of inventory costing. The CFO will use your...
-
Suggest explanations for the origins of "ibu," "pro," and "fen" in the name ibuprofen. Provide a systematic name for thiscompound OH O,N. NO2 NO2 Picric acid
-
The pK a for the picric acid is 0.42. Explain why it is such a strong acid.
-
To find a base that is strong enough to deprotonate benzoic acid but not p-methyl phenol. Then explain how this base might be used to separate these two compounds in the laboratory.
-
What role do social networks and interpersonal relationships play in both perpetuating and challenging existing social structures ?
-
A homeowner is thinking about buying an electric heat pump to save on heating costs. The heat pump is said to reduce fuel consumption by 10 MMBtu per year for 20 years. It costs $1,400 upfront and...
-
How do globalization and technological advancements impact the configuration of social structures, particularly in terms of power dynamics and inequalities ?

Study smarter with the SolutionInn App


