Consider the three constitutional isomers of dioxane (C 4 H 8 O 2 ): One of these
Question:
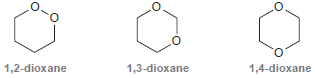
One of these constitutional isomers is stable under basic conditions as well as mildly acidic conditions and is therefore used as a common solvent. Another isomer is only stable under basic conditions but undergoes hydrolysis under mildly acidic conditions. The remaining isomer is extremely unstable and potentially explosive. Identify each isomer, and explain the properties of each compound.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





