Draw a plausible mechanism for each of the following transformations: (a) (b) [TSOH] MENH2 -H20
Question:
Draw a plausible mechanism for each of the following transformations:
(a)
![[TSOH] MENH2 -H20](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1606/7/3/1/2195fc4c5d334dde1606731218633.jpg)
(b)
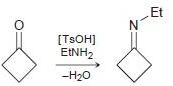
Transcribed Image Text:
[TSOH] MENH2 -H20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (20 reviews)
For each of the mechanisms shown below the first two steps can be reversed first the am...View the full answer

Answered By

John Aketch
I am a dedicated person with high degree of professionalism, particularly in academic writing. My desire is to is to make students excel in their academic endeavor.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw a plausible mechanism for each of the following reactions: (a) (b) [H,SO4] -H20 [H,SO4] -H20
-
Draw a plausible mechanism for each of the following transformations: a. b. c. d. e. Pyridine CI
-
Propose a plausible mechanism for each of the following reactions: a. b. stitl. [H,SO,] Conc. H2SO4
-
Lombard Ltd has been offered a contract for which there is available production capacity. The contract is for 20,000 identical items, manufactured by an intricate assembly operation, to be produced...
-
When writing technical reports, be aware that the numbers, formulas, and concepts can sometimes be overwhelming for the reader. Illustrate how to meet that challenge by doing the following: Identify...
-
Find t such that -/2 t /2 and t satisfies the stated condition. sin t = sin(3/4)
-
The handrails were then sent to the drilling machine where the holes were automatically spaced and drilled by the operator on an industrial quality drill press. Occasionally the operator inspects the...
-
Youve been hired to perform an audit of Hubbard Company for the year ended December 31, 2016. You find the following account balances related to shareholders equity': Preferred stock, $100 par ....$...
-
To: Anthony Carlisle From: Pete Mitchell Date: January 26, Year 1 RE: Audit of Goose, Inc. Tony, I am currently engaged in the audit of the accounts receivable balance of Goose, Incorporated. Here...
-
Determine the enthalpy of combustion of methane (CH4) at 25oC and 1 atm, using the enthalpy of formation data from Table A-26. Assume that the water in the products is in the liquid form. Compare...
-
Draw a plausible mechanism for each of the following transformations: (a) (b) [TSOH] MENH2 -H20 Et [TSOH] EENH2 -H20
-
Using what you know about the expanded source table, fill in the missing values in the table shown here: df Source SS MS Gender 248.25 Parenting style 84.34 33.60 Gender X style Within 1107.2 36...
-
Understand how projects are selected. AppendixLO1
-
Exercise 11-5 Profit allocation in a partnership LO3 Dallas and Weiss formed a partnership to manage rental properties, by investing $198,000 and $242,000, respectively. During its first year, the...
-
Reading following articles and answer the questions: https://www.afr.com/technology/ai-is-coming-for-white-collar-jobs-gates-warns-20230123-p5cev7...
-
1. Citing an example in each case, briefly explain four types of book keeping errors which are not disclosed by trial balance 2. The trial balance extracted from the books of james as at 30 september...
-
Use the universal gravitation formula to determine which object has a larger effect on the Earth's motion through space: the Sun or the Moon. Explain how you are determining this, including very...
-
Pro Cycling Shop is a medium-size seller of the high-end bicycle. Since starting the company 15 years ago, Pro Cycling Shop has been a competitive company across Sarawak, Brunei, Kalimantan, and...
-
Solve each system. If a system is inconsistent or has dependent equations, say so. -5x + 2y = -4 6x + 3y = -6
-
Pappa's Appliances uses the periodic inventory system. Details regarding the inventory of appliances at January 1, purchases invoices during the year, and the inventory count at December 31 are...
-
Classify each of the following compounds as an alkane, alkene, alkyne, alcohol, aldehyde, amine, and so forth. (a) (b) CH3-C¡CH (c) (d) (e) (f) Obtained from oil of cloves -2. Sex attractant...
-
Boron trifluoride (BF3) has no dipole moment ( = 0 D). Explain how this observation confirms the geometry of BF3 predicted by VSEPR theory.
-
Identify all of the functional groups in each of the following compounds: (a) (b) (c) (d) (e) (f) (g) Vitamin D3 HO OMe Aspartame O NH2 NH2 Amphetamine Me Cholesterol HO OCH2CH3 Demerol CH A...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...
-
Yard Professionals Incorporated experienced the following events in Year 1, its first year of operation: Performed services for $31,000 cash. Purchased $7,800 of supplies on account. A physical count...

Study smarter with the SolutionInn App


