In each of the following compounds, identify all carbon atoms that you expect will be deficient in
Question:
a.
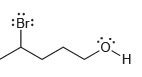
b.
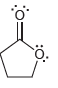
c.
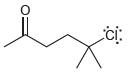
Refer section 1.5
€¢ Bonds are classified as (1) covalent, (2) polar covalent, or (3) ionic.
€¢ Polar covalent bonds exhibit induction, causing the formation of partial positive charges (δ+) and partial negative charges (δ-). Electrostatic potential maps present a visual illustration of partial charges.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





