Question: Learning to extract structural information from molecular formulas: a) Write out the molecular formula for each of the following compounds: Compare the molecular formulas for
Learning to extract structural information from molecular formulas:
a) Write out the molecular formula for each of the following compounds:
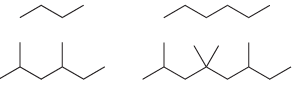
Compare the molecular formulas for the above compounds and fill in the blanks in the following sentence: The number of hydrogen atoms is equal to __________ times the number of carbon atoms, plus __________.
b) Now write out the molecular formula for each of these compounds:
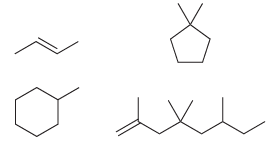
Each of the compounds above has either a double bond or a ring. Compare the molecular formulas for each of these compounds. In each case, the number of hydrogen atoms is __________ times the number of carbon atoms. Fill in the blank.
c) Now write out the molecular formula for each of these compounds:
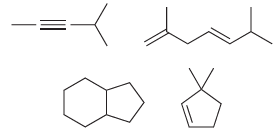
Each of the compounds above has either a triple bond or two double bonds or two rings or a ring and a double bond. Compare the molecular formulas for each of these compounds. In each case, the number of hydrogen atoms is __________ times the number of carbon atoms minus __________. Fill in the blanks.
d) Based on the trends above, answer the following questions about the structure of a compound with molecular formula C24H48. Is it possible for this compound to have a triple bond? Is it possible for this compound to have a double bond?
e) Draw all constitutional isomers that have the molecular formula C4H8.
Step by Step Solution
3.26 Rating (170 Votes )
There are 3 Steps involved in it
a In each of the compounds above the number of hyd... View full answer

Get step-by-step solutions from verified subject matter experts


