The OH group on the side chain of serine is not deprotonated at a pH of 12.
Question:
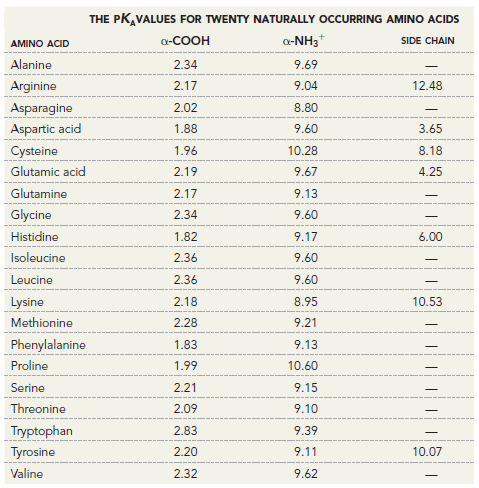
Transcribed Image Text:
THE PK,VALUES FOR TWENTY NATURALLY OCCURRING AMINO ACIDS a-NH3* a-COOH SIDE CHAIN AMINO ACID Alanine 2.34 9.69 Arginine 2.17 9.04 12.48 Asparagine 2.02 8.80 Aspartic acid 1.88 9.60 3.65 Cysteine 1.96 10.28 8.18 Glutamic acid 9.67 2.19 4.25 Glutamine 9.13 2.17 Glycine 2.34 9.60 Histidine 9.17 1.82 6.00 Isoleucine 2.36 9.60 2.36 9.60 Leucine 8.95 Lysine 2.18 10.53 Methionine 9.21 2.28 Phenylalanine 1.83 9.13 Proline 10.60 1.99 Serine 2.21 9.15 Threonine 2.09 9.10 Tryptophan 2.83 9.39 Tyrosine 2.20 9.11 10.07 Valine 2.32 9.62 T|||||||
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (11 reviews)
Tyrosine possesses a phenolic proton which is more readily depro...View the full answer

Answered By

Jane Thuita
I am a highly motivated and experienced tutor with a passion for helping students succeed. I have a strong background in a variety of academic subjects, including mathematics, science, and English. I have experience working with students of all ages and abilities, and I am able to customize my teaching methods to best fit each student's unique learning style. I am also able to work with students who have learning disabilities and those who are English language learners. I am patient, understanding, and dedicated to helping my students reach their full potential. In my free time, I enjoy reading, playing sports, and spending time with my family. I am excited to work with you and help you achieve your goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The citric acid cycle is a series of biological reactions that plays a central role in cell metabolism. The cycle includes dehydration reactions of both malic and citric acids, yielding fumaric and...
-
There are a number of conserved sequences found in an mRNA which dictate where splicing occurs. Where are these sequences found relative to the exon/intron junctions? What is the significance of...
-
Histidine possesses a basic side chain which is protonated at physiological pH. Identify which nitrogen atom in the side chain is protonated.
-
Once down to about 15, the worlds only wild flock of whooping cranes now numbers a record 237 birds in its Texas Coastal Bend wintering ground (www.SunHerald.com). The average whooping crane egg...
-
Marcel Company projects the following sales for the first three months of the year: $11,200 in January; $12,300 in February; and $11,100 in March. The company expects 60% of the sales to be cash and...
-
For short lines less than $80 \mathrm{~km}$ long, loadability is limited by the thermal rating of the conductors or by terminal equipment ratings, not by voltage drop or stability considerations.(a)...
-
Refer to Problem 3.1. Data From Problem 3.1 Consider the National Football League data in Table B.1. a. Find a $95 % \mathrm{CI}$ on $\beta_{7}$. b. Find a $95 %$ CI on the mean number of games won...
-
Prepare all journal entries (budgetary and actual) required in all funds and the GCA-GLTL accounts to record the following transactions and events: 1. The county sold old equipmentoriginal cost...
-
. An urn has 10 balls that are identical except that7are white and 3 are red. A sample of 8 is selected randomly without replacement. What is the probability that exactly 6 are white and2are red?...
-
1. After reviewing her personal automobile policy, Bronwyn realized that she had $75,000 of single-limit, Part A coverage; $15,000 of Part B coverage; $75,000 of Part C coverage; and "full" Part D...
-
Using the data in the following table, calculate the pI of the following amino acids. (a) Aspartic acid (b) Leucine (c) Lysine (d) Proline THE PK,VALUES FOR TWENTY NATURALLY OCCURRING AMINO ACIDS...
-
Explain why it is inappropriate to use a chiral catalyst in the preparation of glycine.
-
Use Table B.3 and find the compressibility of carbon dioxide at the critical point.
-
Describe strategies for effective group writing.
-
Explain and apply positive and otheroriented tone in business messages.
-
Apply principles for writing effective emails.
-
Develop your primary message and key points in the AIM planning process.
-
Describe the interpersonal communication process and barriers to effective communication.
-
A golf ball is hit from the tee and travels a distance of 300 yards. Estimate the magnitude of the impulse imparted to the golf ball. Ignore air drag in your analysis. You will need to estimate the...
-
After looking at the resources, explain what a spirit image is. Why might looking at a god and/or a human in terms of their spirit be helpful if you want to eliminate some of the divisions between...
-
In the addition of just 1 mole of bromine to 1 mole of hex-1-yne, should the hex-1-yne be added to a bromine solution or should the bromine be added to the hex-1-yne? Explain your answer.
-
Propose a mechanism for the entire reaction of pent-1-yne with 2 moles of HBr. Show why Markovnikov's rule should be observed in both the first and second additions of HBr.
-
Predict the major product(s) of the following reactions: (a) phenylacetylene + 2 HBr (b) hex-1-yne + 2 HBr (c) cyclooctyne + 2 HCl (d) hex-2-yne + 2 HCl + 2 HBr
-
(a) Given that z =2+23i and z =-5+5i. Express, and in polar form. (b) Find z and in exponential form. (c) Find (5)*() in the forms of x+yi.
-
Evaluate the impact of mobile banking on traditional revenue streams and business models of banks, such as transaction fees, interchange fees, and overdraft charges. How do banks monetize mobile...
-
1. Researchers wanted to see how two new varieties of fish food compare to a commonly used food. They took 180 similar fish and assigned them randomly to three different tanks. The fish in tank 1...

Study smarter with the SolutionInn App


