Trans-1,3-Dichlorocyclobutane has a measurable dipole moment. Explain why the individual dipole moments of the C-Cl bonds do
Question:
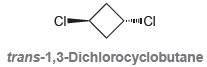
Transcribed Image Text:
CI- trans-1,3-Dichlorocyclobutane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
Cyclobutene adopts a slightly puckered conformation in order to allev...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Trans-1,3-Dibromocyclobutane has a measurable dipole moment. Explain how this proves that the cyclobutane ring is not planar.
-
Which compound has the greater dipole moment? a. b. c. Cl C-C C or CI CI CI C-C or - CH3 CI CH3 CI - or - CH3
-
For each pair of compounds, predict which one has the higher molecular dipole moment, and explain your reasoning. (a) Ethyl chloride or ethyl iodide (b) 1-bromopropane or cyclopropane (c) Cis-2,...
-
Let a = (123) (45) = S, and b = (23) (14) = S5, then aba is equal to (13) (25) (135) (24) (15) (23) (123) (45)
-
What is the current membership of the European Monetary Union (EMU)? How successful has the transition to a single currency been?
-
Using real-world examples, evaluate the arguments for protectionism.
-
Provide examples of Web services and discuss the contribution of Web services to the efficiency of information systems.
-
You are considering opening a copy service in the student union. You estimate your fixed cost at $15,000 and the variable cost of each copy sold at $.01. You expect the selling price to average $.05....
-
Compare the differences between adiabatic, isochoric, isobaric, and isothermal processes
-
What was Lakesides gross profit for January? a. $7,500 b. $ 0 c. $1,900 d. $5,600
-
In each reaction, identify the Lewis acid and the Lewis base: (a) (b) (c) F L-
-
Compound A has molecular formula C 5 H 10 . Hydroboration-oxidation of compound A produces a pair of enantiomers, compounds B and C. When treated with HBr, compound A is converted into compound D,...
-
Janet purchased her personal residence in 2009 for $250,000. In January 2019 she converted it to rental property. The fair market value at the time of conversion was $210,000. a. Determine the amount...
-
Ryan and Peggy are going to a circus. They start at point eight and walk 220 m southeast appointment. They make it right and walk another 220 M. Southwest to the circus at point c. What is their...
-
Whiskey Corporation (the Company) files a federal income tax return and a tax return in one state that has a 10% rate. Assuming that all the Company's sales are allocated to that one state, calculate...
-
If I1= 483 (mA) then what is the magnitude of the magnetic flux through the loop due to the magnetic field arising from the long straight wire? Express your answer in pico-Webers to one place after...
-
A punt is a type of boat that was once used in industrial duck hunting. A punt gun would be attached to the boat and be fired horizontally to kill many birds with one shot.A punt gun fires a kg shell...
-
If an object in uniform circular motion at a radius r , and a velocity v , has a centripetal acceleration of 2 0 m / s ^ 2 . What will the acceleration be if the velocity becomes 2 X as big. ( v > >...
-
Maddox Co. pays salaries monthly on the last day of the month. The following information is available from Maddox Co. for the month ended December 31, Year 1. Administrative salaries...
-
What is the expected payoff of an investment that yields $5,000 with a probability of 0.15 and $500 with a probability of 0.85? Select one: O a. $325 O b. $5,500 O c. $2,750 O d. $1,175
-
Explain which stereo isomer is more stable. Problems using online Three-Dimensional molecular models
-
Explain which isomer has more strain energy in the conformation shown. Problems using online Three-Dimensional molecular models
-
For these compounds, indicate whether the substituents are cis or Trans, whether they are axial or equatorial, whether the conformation shown or the other chair conformation is more stable, and...
-
Excerpts from Andre Company's December 31, 2024 and 2023, financial statements are presented below: Accounts receivable Inventory Net sales Cost of goods sold Total assets Net income Total...
-
The York City Hospital has just acquired new equipment. The equipment cost $ 4 , 2 5 0 , 0 0 0 , and the organization spent $ 1 3 5 , 0 0 0 on upgrading the physical plant to the new equipment will...
-
Calculate the following showing all the necessary steps: Note: CPP rate to be used in the calculation is 5.95%. Note: Don't forget to deduct pay period exemption\ \ Tanya earns $25.00 per hour. This...

Study smarter with the SolutionInn App


