Question: We saw a general rule that the two protons of a CH 2 group will be chemically equivalent if there are no chirality centers in
We saw a general rule that the two protons of a CH2group will be chemically equivalent if there are no chirality centers in the compound. An example of an exception is 3-bromopentane. This compound does not possess a chirality center. Nevertheless, the two highlighted protons are not chemically equivalent. Explain.
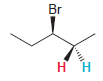
Br
Step by Step Solution
★★★★★
3.42 Rating (158 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The presence of the bromine atom does not render C 3 a chirality c... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


