Question: When methyloxirane is treated with HBr, the bromide ion attacks the less substituted position. However, when phenyloxirane is treated with HBr, the bromide ion attacks
When methyloxirane is treated with HBr, the bromide ion attacks the less substituted position. However, when phenyloxirane is treated with HBr, the bromide ion attacks the more substituted position.
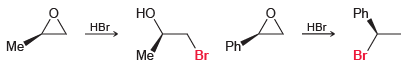
Explain the difference in regiochemistry in terms of a competition between steric effects and electronic effects.
Ph. HBr HBr Me Me Br Br Ph
Step by Step Solution
★★★★★
3.46 Rating (166 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

When methyloxirane is treated with HBr the regiochemical o... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


