Question: Given that the cell does positive work on the electrons, why is it that the work in both energy diagrams in Figure 26.17 is negative?
Given that the cell does positive work on the electrons, why is it that the work in both energy diagrams in Figure 26.17 is negative?
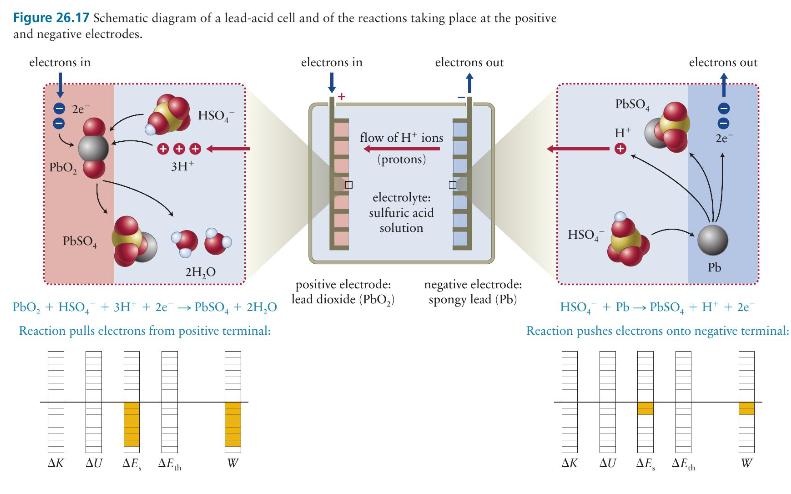
Figure 26.17 Schematic diagram of a lead-acid cell and of the reactions taking place at the positive and negative electrodes. electrons in 2e 3H+ PbSO4 HSO electrons in electrons out flow of H+ ions (protons) electrolyte: sulfuric acid solution 2H,O PbO, + HSO + 3H + 2e PbSO + 2H,O Reaction pulls electrons from positive terminal: positive electrode: lead dioxide (PbO) AK AU AE, AF W HSO PbSO4 H electrons out - 00 2e Pb negative electrode: spongy lead (Pb) HSO + Pb-PbSO, +H+2e Reaction pushes electrons onto negative terminal: AK AE, AF W
Step by Step Solution
3.38 Rating (151 Votes )
There are 3 Steps involved in it
The work is negative because the system under considera... View full answer

Get step-by-step solutions from verified subject matter experts


