Question: If you begin at state 1 again but this time go through the cycle in Figure 21.28 clockwise rather than counterclockwise, what are the magnitude
If you begin at state 1 again but this time go through the cycle in Figure 21.28 clockwise rather than counterclockwise, what are the magnitude and sign of \((a)\) the work done on the system and \((b)\) the work done on the environment?
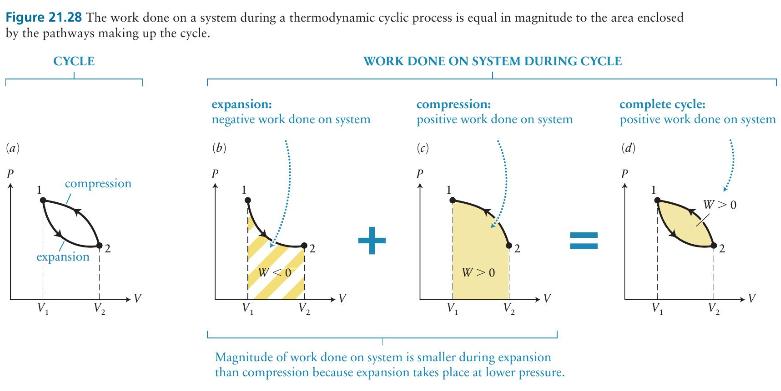
Figure 21.28 The work done on a system during a thermodynamic cyclic process is equal in magnitude to the area enclosed by the pathways making up the cycle. CYCLE WORK DONE ON SYSTEM DURING CYCLE (a) expansion: compression: complete cycle: negative work done on system positive work done on system positive work done on system (b) (c) (d) P P P compression W 0 expansion T V WO V + W 0 Magnitude of work done on system is smaller during expansion than compression because expansion takes place at lower pressure.
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
a Now the area under the expansion path 1 2 is gre... View full answer

Get step-by-step solutions from verified subject matter experts


