In a reversible heat engine, (6.02 times 10^{23}) molecules of nitrogen gas are used as the working
Question:
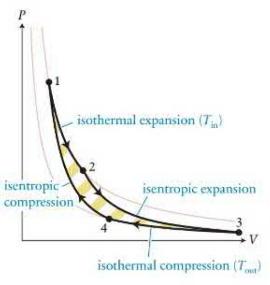
In a reversible heat engine, \(6.02 \times 10^{23}\) molecules of nitrogen gas are used as the working substance. The gas undergoes a Carnot cycle as illustrated in Figure 21.43. The volume of the gas is \(6.00 \times 10^{-3} \mathrm{~m}^{3}\) in state 1 and \(14.0 \times 10^{-3} \mathrm{~m}^{3}\) in state 2 . The low-temperature reservoir is at \(320 \mathrm{~K}\). How much energy is transferred thermally to the low-temperature reservoir during one cycle?
Data from Figure 21.43
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





