Question: A gas containing methane, ethane, and carbon dioxide is analyzed with a gas chromatograph (GC) and flame ionization detector (FID): the GC separates the components
A gas containing methane, ethane, and carbon dioxide is analyzed with a gas chromatograph (GC) and flame ionization detector (FID): the GC separates the components of the gas, and the FID registers signals proportional to the amount of each hydrocarbon (but not CO2) in its sample chamber.
The output of the FID is as follows:
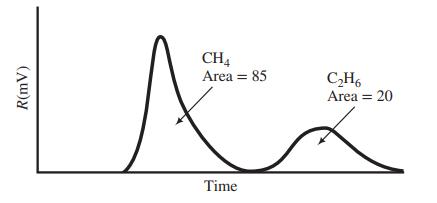
The area under each peak is proportional to the number of carbon atoms in the sample, so that 1 mol of ethane would yield a peak with twice the area of a peak corresponding to 1 mol of methane.
This fuel is being burned with air in a continuous combustion chamber. The molar feed ratio of air to fuel was supposed to be 7:1, but you suspect the air flowmeter is not functioning properly. To check it, you take a 0.50-mol sample of the product gas and pass it through a condenser, which condenses essentially all of the water in the sample. The condensate (which can be assumed to be pure water) is weighed and found to have a mass of 1.134 g. The dry gas leaving the condenser is analyzed and found to contain no hydrocarbons, no CO, and 11.9% CO2.
(a) Calculate the molar composition (component mole fractions) of the fuel gas and the desired percent excess air.
(b) Calculate the actual molar feed ratio of air to fuel and the actual percent excess air.
CH4 Area = 85 C,H6 Area = 20 Time R(mV)
Step by Step Solution
3.37 Rating (153 Votes )
There are 3 Steps involved in it
a To calculate the molar composition component mole fractions of the fuel gas and the desired percen... View full answer

Get step-by-step solutions from verified subject matter experts


