Question: A liquid mixture containing 50 mole% propane, 30% n-butane, and 20% isobutane is stored in a rigid container at 77F. The container has a maximum
A liquid mixture containing 50 mole% propane, 30% n-butane, and 20% isobutane is stored in a rigid container at 77°F. The container has a maximum allowable working pressure of 400 psig. The head space above the liquid contains only vapors of the three hydrocarbons.
(a) A form of the Antoine equation for which constants for the three components are available is log10p* = A - B/(T + C) where p∗ is in bar and T is in kelvin. The constants and the data range from which they were obtained are given in the following table:
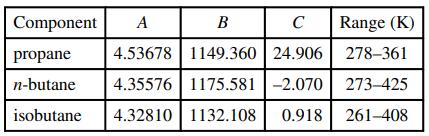
Using these values and Raoult’s law, show that use of the container at the given temperature is safe.
(b) Assume that upon heating there is little change in the liquid composition, and obtain a rough estimate of the temperature above which the maximum allowable pressure would be exceeded. Explain why the assumption regarding no change in liquid composition is reasonable.
Component A B Range (K) propane 4.53678 1149.360 24.906 278-361 n-butane 4.35576 1175.581 -2.070 273-425 isobutane 4.32810 | 1132.108 0.918 261-408
Step by Step Solution
3.47 Rating (160 Votes )
There are 3 Steps involved in it
Given temperature 77 0 F 298 K Lets denote propane by ... View full answer

Get step-by-step solutions from verified subject matter experts


