At 300 K, the partial vapour pressures of HCl (that is, the partial pressure of the HCl
Question:
At 300 K, the partial vapour pressures of HCl (that is, the partial pressure of the HCl vapour) in liquid GeCl4 are as follows:
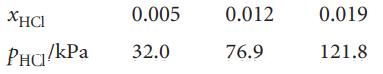
Show that the solution obeys Henry’s law in this range of mole fractions, and calculate Henry’s law constant at 300 K.
Transcribed Image Text:
ХНСI PHO/kPa 0.005 32.0 0.012 76.9 0.019 121.8
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
Henrys law states that the partial pressure of a gas in a liquid is directly propor...View the full answer

Answered By

Dansteve Matoke
As a consequence of more than three years of experience in ACADEMIC WRITING, I have vast, diverse knowledge and impeccable grammar. I guarantee the quality of my work across multiple fields. I intend to precisely meet the clients' expectations. Let's work together to achieve exceptional grades.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
At 310 K, the partial vapour pressures of a substance B dissolved in a liquid A are as follows: xB 0.010 0.015 0.020 Pg/kPa 82.0 122.0 166.1 Show that the solution obeys Henry's law in this range of...
-
At 300 K the reaction below obeys the rate law rate=k[NOCl] 2 where k= 2.8x10 -5 M -1 * S -1 2NOCl ------> 2NO + Cl 2 Suppose 1.0 mole of NOCl is introduced into a 2.0- liter container at 300 k....
-
The vapour pressure of pure liquid A at 293 K is 68.8 kPa and that of pure liquid B is 82.1 kPa. These two compounds form ideal liquid and gaseous mixtures. Consider the equilibrium composition of a...
-
Data Set 32 "Airport Data Speeds" in Appendix B includes Sprint data speeds (mbps). The accompanying TI-83 / 84 Plus display results from using those data to test the claim that they are from a...
-
You bought a stock for $40, received a dividend of $1, and sold it for $41 after five months. What is your annualized arithmetic rate of return?
-
By suggesting this is operating cash flow, Wald is operating under: a. Instrumental virtue. b. Rests idea of the character. c. Kohlbergs idea of Law and Order. d. The GVV idea of Locus of Loyalty.
-
The cash flows associated with a project are shown below. The interest rate varies from year to year as shown. Determine an equivalent uniform annual series of cash flows. EOY Cash Flow Interest...
-
The Mead Company uses a perpetual inventory system and engaged in the following transactions during the month of May: Date Transaction_______________________________________________ May 1 Made cash...
-
Estimated total machine-hours used Estimated total fixed manufacturing overhead Estimated variable manufacturing overhead per machine-hour Molding 2,500 $ 12,000 $ 2.20 Fabrication 1,500 $ 16,200 $...
-
Complete the function dictchk creates a new dictionary from an input dictionary as follows. Each key in the input dictionary is checked to see if it contains the character 'k'. If so, the new...
-
How is Raoults law modified so as to describe the vapour pressure of real solutions?
-
The partial molar volumes of acetone (propanone) and chloroform (trichloromethane) in a mixture in which the mole fraction of CHCl 3 is 0.4693 are 74.166 cm 3 mol 1 and 80.235 cm 3 mol 1 ,...
-
How do you get the IP address of a machine from its hostname?
-
Daryll and Sharon Dykes filed a petition for Chapter 7 relief in a federal bankruptcy court, reporting just under $400,000 in assets, over $5.6 million in liabilities, and a monthly income that is...
-
Sue applies for a fire insurance policy for her warehouse from A&I Insurance Company. To obtain a lower premium, she misrepresents the age of the property. The policy is granted. After the warehouse...
-
Ann rents a kayak from Boaters Marina for a days paddling on Clearwater Creek. When Ann takes a break onshore, Donnie steals the kayak. Ann can attempt to a. recover the price of the kayak from...
-
Satellite Communications, Inc., takes out an insurance policy on its plant. For which of the following reasons could the insurer cancel the policy? a. Satellites president appears as a witness in a...
-
Heneli borrows $150,000 from Countywide Credit Union to buy a home. By recording the mortgage, Countywide protects its a. priority against a previously filed lien on the property. b. priority against...
-
Verify that the discrete metric on a set S as defined in 11.4.2(f) is a metric.
-
Using Apple, demonstrate how the differentiation strategy can be well implemented.
-
Given that F=dV/dr, calculate the distance dependence of the force acting between two non-bonded groups of atoms in a polymer chain that have a London dispersion interaction with each other.
-
D.D. Nelson et al. (Science 238, 1670 (1987)) examined several weakly bound gas-phase complexes of ammonia in search of examples in which the H atoms in NH 3 formed hydrogen bonds, but found none....
-
Estimate the energy of the dispersion interaction (use the London formula) for two He atoms separated by 1.0nm. Relevant data can be found in the Resource section.
-
Wanda is reviewing her tax returns from the previous year and is shocked at how much tax she paid the government. She had good income but had to pay a large number of self-employment taxes on top of...
-
Katies Cleaning Service has cleaning contracts for 15 apartments, 45 family homes, and 25 office buildings. She estimates that an apartment takes 4 hours to clean, a home takes 6 hours to clean, and...
-
1. Quikpak sells returnable containers to major food processors. The price received for the containers is 2 per unit. Of this amount 1.25 is profit contribution. Quikpak is considering an attempt to...

Study smarter with the SolutionInn App


