Question: C. Huang and W.P. Cheng (J. Colloid Interface Sci. 188, 270 (1997)) examined the adsorption of the hexacyanoferrate(III) ion, [Fe(CN) 6 ] 3 , on
C. Huang and W.P. Cheng (J. Colloid Interface Sci. 188, 270 (1997)) examined the adsorption of the hexacyanoferrate(III) ion, [Fe(CN)6]3−, on γ-Al2O3 from aqueous solution. They modelled the adsorption with a modified Langmuir isotherm, obtaining the following values of α at pH=6.5:
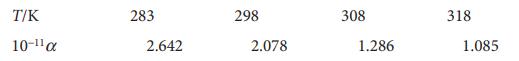
Determine the isosteric enthalpy of adsorption, ΔadsH⦵, at this pH. The researchers also reported ΔadsS⦵ =+146 Jmol−1K−1 under these conditions. Determine ΔadsG⦵.
T/K 10-11 283 2.642 298 2.078 308 1.286 318 1.085
Step by Step Solution
3.37 Rating (172 Votes )
There are 3 Steps involved in it
Calculation The enthalpy of adsorption adsH is given by ... View full answer

Get step-by-step solutions from verified subject matter experts


