Question: Calculate the van der Waals parameters of carbon dioxide from the values of the critical constants and compare your results with the values for and
Table 7.4
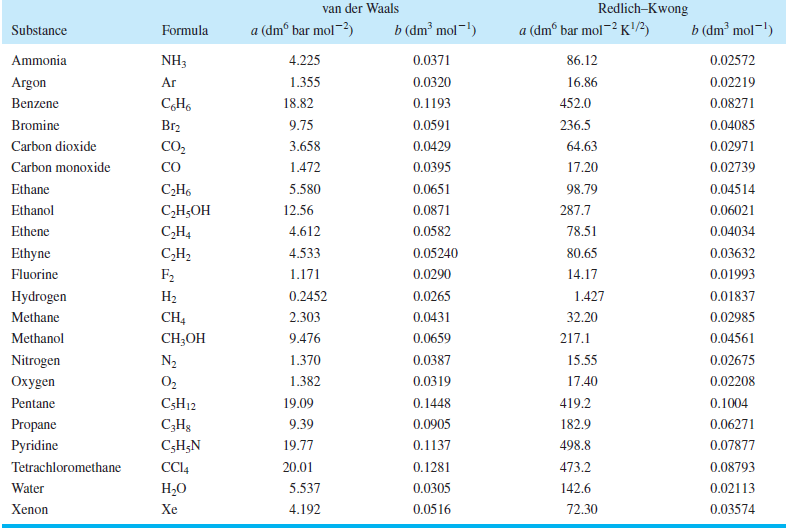
RedlichKwong van der Waals b (dm mol!) a (dm bar mol-) b (dm mol-!) a (dm bar mol~ K) Substance Formula NH3 0.0371 Ammonia 4.225 86.12 0.02572 Argon Ar 1.355 0.0320 16.86 0.02219 452.0 Benzene 18.82 0.1193 0.08271 Br2 9.75 236.5 Bromine 0.0591 0.04085 CO2 0.0429 Carbon dioxide 3.658 64.63 0.02971 CO Carbon monoxide 1.472 0.0395 17.20 0.02739 0.04514 Ethane -. 5.580 0.0651 98.79 Ethanol -, 12.56 0.0871 287.7 0.06021 Ethene C,H4 4.612 0.0582 78.51 0.04034 CH2 F2 0.05240 0.03632 Ethyne 4.533 80.65 Fluorine 1.171 0.0290 14.17 0.01993 1.427 Hydrogen 0.2452 0.0265 0.01837 CH4 CH-O 2.303 0.0431 Methane 32.20 0.02985 0.0659 Methanol 9.476 217.1 0.04561 Nitrogen 0.02675 N2 1.370 0.0387 15.55 0.0319 gen O2 1.382 17.40 0.02208 Pentane C5H12 19.09 0.1448 419.2 0.1004 Propane C;H3 9.39 0.0905 182.9 0.06271 Pyridine C;H;N 19.77 0.1137 498.8 0.07877 Tetrachloromethane CC4 20.01 0.1281 473.2 0.08793 Water . 5.537 0.0305 142.6 0.02113 4.192 Xenon Xe 0.0516 72.30 0.03574
Step by Step Solution
3.48 Rating (181 Votes )
There are 3 Steps involved in it
We use the values for the critical cons... View full answer

Get step-by-step solutions from verified subject matter experts


