Carbon nanotubes (CNT) are among the most versatile building blocks in nanotechnology. These unique pure carbon materials
Question:
Carbon nanotubes (CNT) are among the most versatile building blocks in nanotechnology. These unique pure carbon materials resemble rolled-up sheets of graphite with diameters of several nanometers and lengths up to several micrometers. They are stronger than steel, have higher thermal conductivities than most known materials, and have electrical conductivities like that of copper but with higher currentcarrying capacity. Molecular transistors and biosensors are among their many applications.
While most carbon nanotube research has been based on laboratory-scale synthesis, commercial applications involve large industrial-scale processes. In one such process, carbon monoxide saturated with an organo-metallic compound (iron penta-carbonyl) is decomposed at high temperature and pressure to form CNT, amorphous carbon, and CO2. Each “molecule” of CNT contains roughly 3000 carbon atoms. The reactions by which such molecules are formed are:
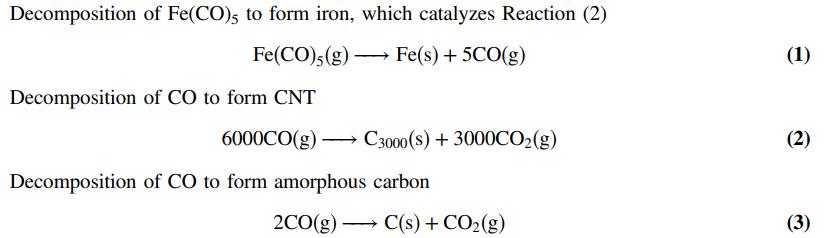
In the process to be analyzed, a fresh feed of CO saturated with Fe(CO)5(v) contains 19.2 wt% of the latter component. The feed is joined by a recycle stream of pure CO and fed to the reactor, where all of the iron penta-carbonyl decomposes. Based on laboratory data, 20.0% of the CO fed to the reactor is converted, and the selectivity of CNT to amorphous carbon production is (9.00 kmol CNT/kmol C). The reactor effluent passes through a complex separation process that yields three product streams: one consists of solid CNT, C, and Fe; a second is CO2 ; and the third is the recycled CO. You wish to determine the flow rate of the fresh feed (SCM/h), the total CO2 generated in the process (kg/h), and the ratio (kmol CO recycled/kmol CO in fresh feed).
(a) Take a basis of 100 kmol fresh feed. Draw and fully label a process flow chart and do degree-offreedom analyses for the overall process, the fresh-feed/recycle mixing point, the reactor, and the separation process. Base the analyses for reactive systems on atomic balances.
(b) Write and solve overall balances, and then scale the process to calculate the flow rate (SCM/h) of fresh feed required to produce 1000 kg CNT/h and the mass flow rate of CO2 that would be produced.
(c) In your degree-of-freedom analysis of the reactor, you might have counted separate balances for C (atomic carbon) and O (atomic oxygen). In fact, those two balances are not independent, so one but not both of them should be counted. Revise your analysis if necessary, and then calculate the ratio (kmol CO recycled/kmol CO in fresh feed).
(d) Prove that the atomic carbon and oxygen balances on the reactor are not independent equations.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





