Question: Consider the reaction TiO 2 (s) + 2 C(graphite) + 2 Cl 2 (g) 2 CO(g) + TiCl 4 (l) for which ÎH o R
Consider the reaction TiO2(s) + 2 C(graphite) + 2 Cl2(g) †’ 2 CO(g) + TiCl4(l) for which ΔHoR ,298 K= €“80. kJ mol€“1. Given the following data at 25°C,
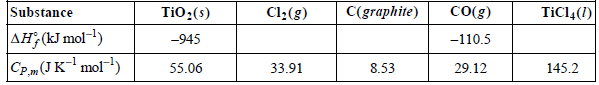
Assume that the heat capacities are independent of temperature.
a. Calculate ΔHoR at 135.8ºC, the boiling point of TiCl4.
b. Calculate ΔHof for TiCl4(l) at 25ºC.
C(graphite) Co(g) TiCl4(1) TiO:(s) Cl:(g) Substance AH, (kJ mol-1) -110.5 -945 55.06 8.53 29.12 33.91 145.2 CP.(JKmol)
Step by Step Solution
3.50 Rating (167 Votes )
There are 3 Steps involved in it
a In this case the heat capacities are assumed to be in... View full answer

Get step-by-step solutions from verified subject matter experts


