Dialysis may be used to study the binding of small molecules to macromolecules, such as an inhibitor
Question:
Dialysis may be used to study the binding of small molecules to macromolecules, such as an inhibitor to an enzyme, an antibiotic to DNA, and any other instance of cooperation or inhibition by small molecules attaching to large ones. To see how this is possible, suppose inside the dialysis bag the molar concentration of the macromolecule M is [M] and the total concentration of small molecule A is [A]in. This total concentration is the sum of the concentrations of free A and bound A, which we write [A]free and [A]bound, respectively. At equilibrium, μA,free=μA,out, which implies that [A]free=[A]out, provided the activity coefficient of A is the same in both solutions. Therefore, by measuring the concentration of A in the solution outside the bag, we can find the concentration of unbound A in the macromolecule solution and, from the difference [A]in−[A]free=[A]in−[A]out, the concentration of bound A. Now we explore the quantitative consequences of the experimental arrangement just described.
(a) The average number of A molecules bound to M molecules, ν, is
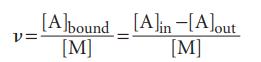
The bound and unbound A molecules are in equilibrium, M+A ⇌ MA. Recall from introductory chemistry that we may write the equilibrium constant for binding, K, as
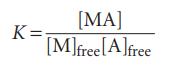
Now show that
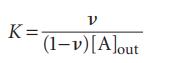
(b) If there are N identical and independent binding sites on each macromolecule, each macromolecule behaves like N separate smaller macromolecules, with the same value of K for each site. It follows that the average number of A molecules per site is ν/N. Show that, in this case, we may write the Scatchard equation:
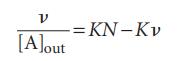
(c) To apply the Scatchard equation, consider the binding of ethidium bromide (E−) to a short piece of DNA by a process called intercalation, in which the aromatic ethidium cation fits between two adjacent DNA base pairs. An equilibrium dialysis experiment was used to study the binding of ethidium bromide (EB) to a short piece of DNA. A 1.00µmoldm−3 aqueous solution of the DNA sample was dialysed against an excess of EB. The following data were obtained for the total concentration of EB:
[EB]/(μmoldm−3)
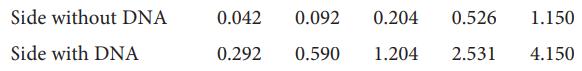
From these data, make a Scatchard plot and evaluate the intrinsic equilibrium constant, K, and total number of sites per DNA molecule. Is the identical and independent sites model for binding applicable?
Step by Step Answer:

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula





