Draw and label the given streams and derive expressions for the indicated quantities in terms of labeled
Question:
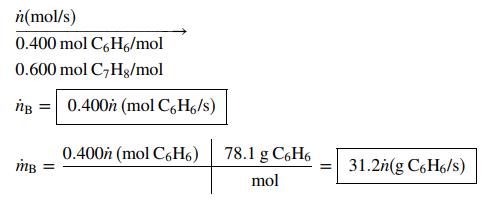
Draw and label the given streams and derive expressions for the indicated quantities in terms of labeled variables. The solution of Part (a) is given as an illustration.
(a) A continuous stream contains 40.0 mole% benzene and the balance toluene. Write expressions for the molar and mass flow rates of benzene, ṅB(mol C6H6/s) and ṁB(kg C6H6/s), in terms of the total molar flow rate of the stream, ṅ(mol/s).
(b) The feed to a batch process contains equimolar quantities of nitrogen and methane. Write an expression for the kilograms of nitrogen in terms of the total moles n(mol) of this mixture.
(c) A stream containing ethane, propane, and butane has a mass flow rate of 100.0 g/s. Write an expression for the molar flow rate of ethane, ṅE(lb-mole C2H6/h), in terms of the mass fraction of this species, xE.
(d) A continuous stream of humid air contains water vapor and dry air, the latter containing approximately 21 mole% O2 and 79% N2. Write expressions for the molar flow rate of O2 and for the mole fractions of H2O and O2 in the gas in terms of ṅ1(lb-mole H2O/s) and ṅ2(lb-mole dry air/s).
(e) The product from a batch reactor contains NO, NO2, and N2O4. The mole fraction of NO is 0.400. Write an expression for the gram-moles of N2O4 in terms of n(mol mixture) and yNO2 (mol NO2/mol).
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





