Estimate the vapor pressure of acetone (mm Hg) at 50C (a) from data in Perrys Chemical Engineers
Question:
Estimate the vapor pressure of acetone (mm Hg) at 50°C (a) from data in Perry’s Chemical Engineers’ Handbook (Footnote 1) and the Clausius–Clapeyron equation, (b) from the Antoine equation using parameters from Table B.4, and (c) using the AntoineP function in APEx.
Table B.4
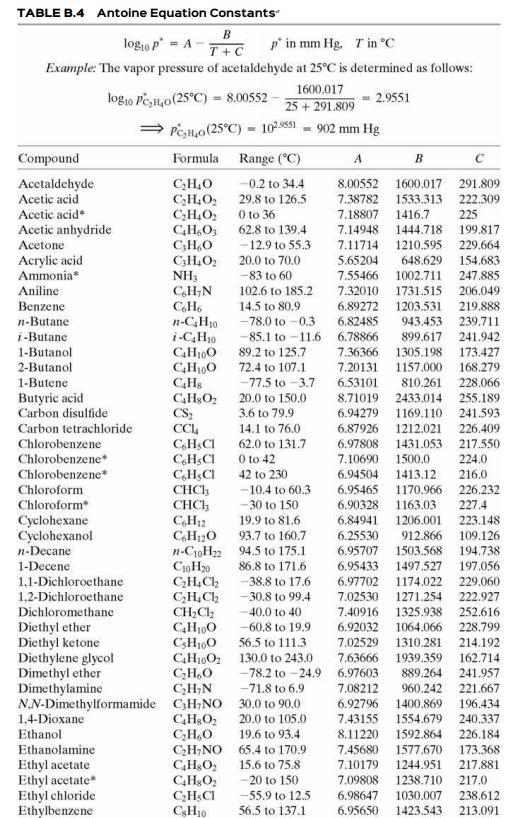
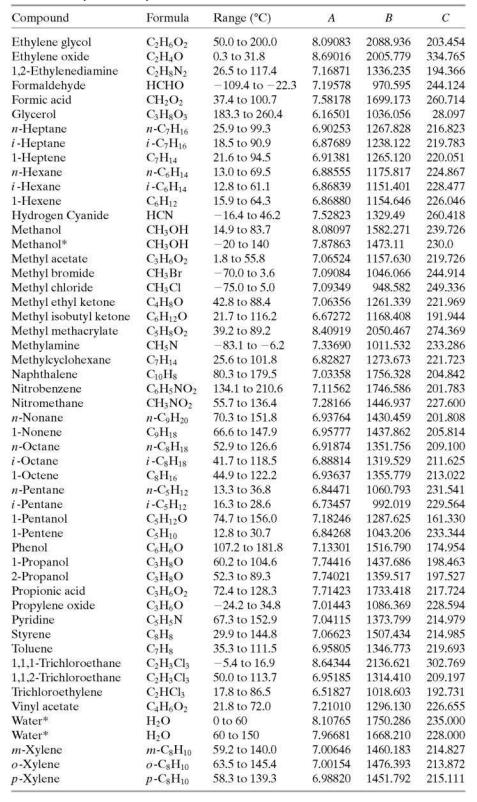
TABLE B.4 Antoine Equation Constants" B log,o p = A p' in mm Hg. Tin °C T+C Example: The vapor pressure of acetaldehyde at 25°C is determined as follows: 1600.017 log10 Pe,H,o(25°C) = 8.00552 25 + 291.809 2.9551 Pen,o(25°C) = 10s51 - 902 mm Hg Compound Formula Range (°C) A B Acctaldehyde Acetic acid C,H,0 C;H,O, C,H,O2 CH,O, C;H,O C3H,O2 NH3 C,H;N -0.2 to 34.4 8.00552 1600.017 291.809 29.8 to 126.5 O to 36 62.8 to 139.4 7.38782 1533.313 222.309 Acetic acid* 7.18807 1416.7 225 Acetic anhydride Acetone 7.14948 1444.718 1210.595 648.629 1002.711 199.817 - 12.9 to 55.3 7.11714 229,664 Acrylic acid Ammonia* 20.0 to 70.0 5.65204 154.683 -83 to 60 7.55466 247.885 Aniline 102.6 to 185.2 7.32010 1731.515 206.049 Benzene 14.5 to 80.9 -78.0 to -0.3 6.89272 1203.531 219.888 n-Butane 6.82485 943.453 n-C,H30 i-C,H10 239.711 i-Butane -85.1 to -11.6 6.78866 89.2 to 125.7 72.4 to 107.1 -77.5 to -3.7 899.617 241.942 1-Butanol 2-Butanol 1-Butene 7.36366 1305.198 173.427 168.279 CH0 CHs 7.20131 1157.000 6.53101 810.261 8.71019 2433.014 1169.110 1212.021 228.066 Butyric acid Carbon disulfide 20.0 to 150.0 3.6 to 79.9 255.189 241.593 CS; CC, C,HSCI C,H;CI C,H;CI CHCH CHCI; C,H12 C,H120 n-C10H2 94.5 to 175.1 CioH20 C;H,Ch 6.94279 Carbon tetrachloride 14.1 to 76.0 6.87926 226.409 Chlorobenzene 62.0 to 131.7 6.97808 1431.053 217.550 Chlorobenzene* 0 to 42 7.10690 1500.0 224.0 Chlorobenzene* 42 to 230 6.94504 1413.12 216.0 Chloroform -10.4 to 60.3 6.95465 1170.966 226.232 Chloroform -30 to 150 6.90328 1163.03 227.4 223.148 Cyclohexane Cyclohexanol 19.9 to 81.6 6.84941 1206.001 93.7 to 160.7 6.25530 912.866 109.126 n-Decane 194.738 6.95707 6.95433 1497.527 1503.568 1-Decene 86.8 to 171.6 197.056 1,1-Dichloroethane 1,2-Dichloroethane Dichloromethane - 38.8 to 17.6 6.97702 1174.022 229.060 -30.8 to 99.4 7.02530 1271.254 222.927 CH;Cl2 C,H100 C;H100 GH1002 130.0 to 243.0 CH,O C;H,N 40.0 to 40 7.40916 1325.938 1064.066 1310.281 252.616 228.799 Diethyl ether Diethyl ketone Diethylene glycol Dimethyl ether Dimethylamine N.N-Dimethylformamide C;H;NO 30.0 to 90.0 -60.8 to 19.9 56.5 to 111.3 6.92032 7.02529 214.192 -78.2 to -24.9 6.97603 -71.8 to 6.9 7.63666 1939.359 889.264 960.242 162.714 241.957 7.08212 221.667 6.92796 1400.869 7.43155 1554.679 196.434 1,4-Dioxane Ethanol C,H&O; CH,O C;H;NO 65.4 to 170.9 20.0 to 105.0 240.337 19.6 to 93.4 8.11220 1592.864 226.184 Ethanolamine 7.45680 1577.670 173.368 Ethyl acetate Ethyl acetate Ethyl chloride Ethylbenzene 15.6 to 75.8 7.10179 1244.951 217.881 -20 to 150 7.09808 1238.710 217.0 C;H;CI CH10 -55.9 to 12.5 6.98647 1030.007 238.612 56.5 to 137.1 6.95650 1423.543 213.091
Step by Step Answer:
Estimation of vapor pressure Vapor pressure is a measure of tendency of material change into the gas...View the full answer



Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
At 300C, the vapor pressure of Hg is 32.97 torr. What mass of Au would have to be dissolved in 5.00 g of Hg to lower its vapor pressure to 25.00 torr?
-
At 300C, the vapor pressure of Hg is 32.97 torr. If 0.775 g of Au were dissolved into 3.77 g of Hg, what would be the vapor pressure of the solution?
-
The Dew Point The vapor pressure of water (see Problem 18.88) decreases as the temperature decreases. If the amount of water vapor in the air is kept constant as the air is cooled, a temperature is...
-
Solve: [1+ log (xy)] dx + [ 1 + x/y ]dy = 0.
-
Pyruvate carboxylase is thought to activate CO2 by ATP, through formation of carboxyphosphate as an intermediate. Propose a mechanism for the formation of this intermediate.
-
Identify which of the accounts listed in the table above are expense accounts and which ones are revenue accounts.
-
Discuss why financial management is important to all types of businesses.
-
Planet Products, Inc., completed Job 2525 on May 31, and there were no jobs in process in the plant. Prior to June 1, the predetermined overhead application rate for June was computed from the...
-
Find the derivative of the function f(x) = {3x^2 + 2x - 5}/{x^2 +1}
-
The Plastic Lumber Company, Inc., (PLC) is a manufacturer that takes in post- consumer plastics (i. e., empty milk jugs) and recycles those plastics into a plastic lumber that can be used to build...
-
Estimate the vapor pressure of acetone (mm Hg) at 50C (a) from data in Perrys Chemical Engineers Handbook (Footnote 1) and the ClausiusClapeyron equation, (b) from the Antoine equation using...
-
The vapor pressure of an organic solvent is 50 mm Hg at 25C and 200 mm Hg at 45C. The solvent is the only species in a closed flask at 35C and is present in both liquid and vapor states. The volume...
-
Why would a company use the sum-of-the-year or declining-balance methods to calculate depreciation?
-
Economic conditions and financial markets were significantly affected by the coronavirus that swept around the world in 2020. A decade earlier, a similar phenomenon was caused by the global financial...
-
Your firm spends $4500 every month on printing and mailing costs, sending statements to customers. If the interest rate is 1.47% per month, what is the present value of eliminating this cost by...
-
You are looking to buy a car and can afford to pay $195 per month. If the interest rate on a car loan is 0.72% per month for a 60-month loan, what is the most expensive car you can afford to buy?
-
In relation to trust distributions to beneficiaries, explain the 65-day rule.
-
How does the Durbin-Watson h adjust in the test for autocorrelation? Under what condition(s) should it be used?
-
In each of the following three nuclear fusion reactions, the masses of the nuclei are given beneath each nucleus. Rank the energy produced by each reaction in descending order (greatest first). (a)...
-
Identify the reagents necessary to accomplish each of the transformations shown below. If you are having trouble, the reagents for these transformations appear on page 444, but you should first try...
-
Identify the reagents you would use to accomplish each of the following transformations: (a) Convert 2-methyl-2-butene into a monosubstituted alkene (b) Convert 2,3-dimethyl-1-hexene into a...
-
Each of the following compounds can be prepared with an alkyl iodide and a suitable nucleophile. In each case, identify the alkyl iodide and the nucleophile that you would use. (a) (b) (c) (d) (e) (f)
-
In October 20X5, Pollock Company exchanged a used packaging machine having a book value of $240,000 for a new machine and paid a cash difference of $30,000. The market value of the used packaging...
-
Project managers should track the details of their projects to be transparent and manage risks as they arise. What is another benefit of tracking in project management?
-
During 2020, Nike disposed of a machine that had been acquired on January 1, 2014 for a purchase price of $20 million. The machine was being depreciated using the straight-line method, a $4 million...

Study smarter with the SolutionInn App


