Question: Explain why all possible wave functions between the fully bonding and the fully anti-bonding are possible for the bands shown in Figure 24.22. Figure 24.22
Figure 24.22
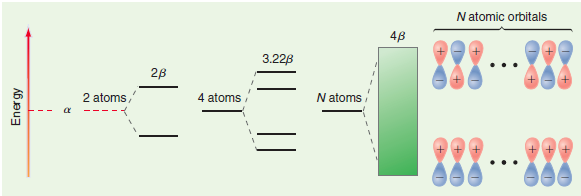
N atomic orbitals 48 3.22B 28 2 atoms, N atoms 4 atoms ... Energy
Step by Step Solution
3.54 Rating (171 Votes )
There are 3 Steps involved in it
A linear molecule containing N atoms will form a pseudomolecule composed of N ... View full answer

Get step-by-step solutions from verified subject matter experts


