In some catalytic reactions the products adsorb more strongly than the reacting gas. This is the case,
Question:
In some catalytic reactions the products adsorb more strongly than the reacting gas. This is the case, for instance, in the catalytic decomposition of ammonia on platinum at 1000 °C. As a first step in examining the kinetics of this type of process, show that the rate of ammonia decomposition should follow in the limit of very strong adsorption of hydrogen.
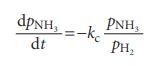
Start by showing that when a gas J adsorbs very strongly, and its pressure is pJ , that the fraction of uncovered sites is approximately 1/KpJ . Solve the rate equation for the catalytic decomposition of NH3 on platinum and show that a plot of F(t)=(1/t) × ln(p/p0) against G(t)=(p−p0)/t, where p is the pressure of ammonia, should give a straight line from which kc can be determined. Check the rate law on the basis of the following data, and find kc for the reaction.

Step by Step Answer:

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula





