Monochlorobenzene (M) is produced commercially by the direct catalytic chlorination of benzene (B) at 40C and 120
Question:
Monochlorobenzene (M) is produced commercially by the direct catalytic chlorination of benzene (B) at 40°C and 120 kPa absolute. In the process, dichlorobenzene (D) is generated as a co-product:
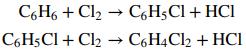
Liquid and gas streams leave the reactor. The liquid contains 49.2 wt% M, 29.6% D, and the remainder unreacted B. The gas, which is sent to a treatment facility, contains 92%(v/v) HCl and 8% unreacted chlorine. Assume ideal-gas behavior.
(a) What volume of gas leaves the reactor (m3/kg B fed)?
(b) The pipe through which the gas is to flow is sized so that the gas velocity is no greater than 10 m/s. Derive an expression relating pipe diameter dp (cm) to benzene feed rate ṁB0 (kg B/min).
(c) In 2004, the demand for monochlorobenzene was projected to increase by 6%/year through the year 2007. What factors were contributing to the increased demand when the projection was made?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





