Recovery of a solvent vapor from a gas stream by condensation can be achieved by cooling the
Question:
Recovery of a solvent vapor from a gas stream by condensation can be achieved by cooling the gas, by compressing it, or by a combination of these operations. The greater the compression, the less cooling is needed.
(a) A gas mixture at a pressure P0 and temperature T0 is the feed to a recovery process. A single condensable vapor and several noncondensable gases are present in the mixture, giving the feed a dew point of Td0. A fraction f of the vapor is to be condensed. For a gas feed rate of ṅ0, draw and label a flowchart. Then derive the following relationship for the final condenser pressure in terms of the final temperature Tf and the specified feed conditions and fractional solvent recovery:
![p*(Tt)[1-fp*(Ta0o)/Po] (1-f)p*(Tao)/Po %3D](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1589/9/8/5/9685ec542b0c03881589985968154.jpg)
Also show that for a given final condenser pressure, the vapor pressure of the condensable component is given by
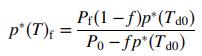
(b) The cost of refrigeration equipment and the compressor can be estimated using the empirical formulas.
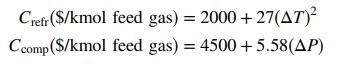
where ΔT(°C) = T0 - Tf and ΔP(mm Hg) = Pf - P0. Prepare a spreadsheet having the form shown below to estimate the cost of a process in which ethyl benzene (also written as ethylbenzene) is recovered from a gaseous mixture of ethyl benzene and nitrogen. You may use APEx functions or the Antoine constants in Table B.4 in your calculations. Explore two regions of operation: The first should vary the final temperature between T1 = 45°C and T10 = 0°C in 5°C decrements, and the second should vary the final pressure between P1 = 1000 mm Hg and P10 = 10;000 mm Hg in 1000 mm Hg increments.
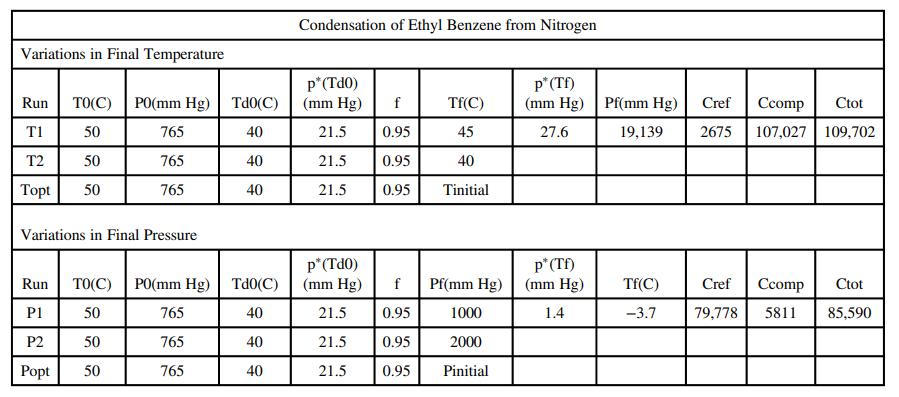
The row shown above for Run T1 contains results for a feed gas at 50°C and 765 mm Hg with a dew point of 40°C, from which 95% of the ethylbenzene is to be recovered by cooling the mixture to 45°C. The output shows that the mixture must be compressed to 19,139 mm Hg to achieve the desired recovery, and that the costs of refrigeration and compression and the total cost ($/kmol feed gas) are, respectively, $2675, $107,027, and $109,702. (We recognize that the significant figures shown are excessive, but leave them in the spreadsheet.)
(c) When you have constructed the spreadsheet and duplicated the results shown for Run T1, copy that row into the next 9 rows and change the values in the first and seventh columns to correspond to the requested run numbers and final temperature. Note what happens to the calculated values of Pf , Crefr, Ccomp, and Ctot as you move from Run T1 to Run T10. Now, copy the row for Run T10 into the next row of the spreadsheet and label it Topt. Use Solver to minimize the total cost by varying the final temperature.
(d) Repeat the procedures from Part (c) but this time vary the final pressure, Pf. Use Solver again to minimize total cost, this time by adjusting Pf . You should obtain the same values for Run Popt as were determined in Run Topt.
(e) Summarize the effects of Tf and Pf on one another and on the refrigeration and compression costs. Explain why the total cost has a minimum.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





