Sodium hydroxide is dissolved in enough water to make up a 20.0 mole% solution. (a) If the
Question:
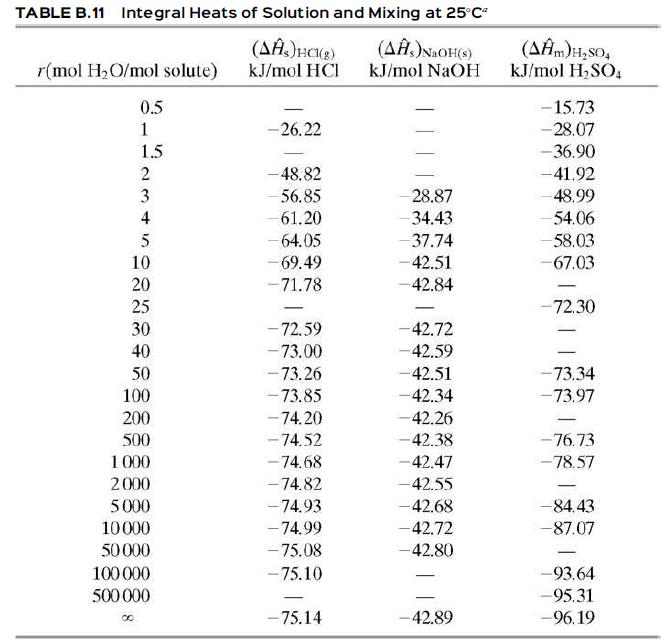
Sodium hydroxide is dissolved in enough water to make up a 20.0 mole% solution.
(a) If the NaOH and water are initially at 77°F (25°C), how much heat (Btu/lb product solution) must be removed for the solution also to be at 77°F. Assume the process is carried out at constant pressure, so that Q = ΔH, and use Table B.11 to evaluate ΔĤs.
(b) If the dissolution is done adiabatically, estimate the final temperature of the solution. Assume that the heat capacity of the solution is approximately that of pure liquid water.
(c) If the process of Part (b) were actually carried out, the final temperature would be less than the value calculated. Why? (Neglect errors caused by the assumptions of adiabatic dissolution and a solution heat capacity equal to that of pure water.)
Table B.11
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





