Question: Starting from the expression C p C V = T(p/T) V (V/T) p , use the appropriate relations between partial derivatives to show that
Starting from the expression Cp − CV = T(∂p/∂T)V(∂V/∂T)p, use the appropriate relations between partial derivatives to show that
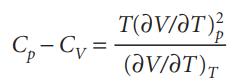
Evaluate Cp − CV for a perfect gas.
C-C= / ) V/T).
Step by Step Solution
3.37 Rating (166 Votes )
There are 3 Steps involved in it
Answer The partial derivatives of PV with respect to pressure are zero heat does not change pressure ... View full answer

Get step-by-step solutions from verified subject matter experts


