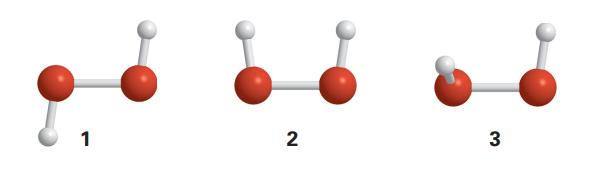
Suppose that three conformations are proposed for the nonlinear molecule H 2 O 2 (1, 2, and
Question:
Suppose that three conformations are proposed for the nonlinear molecule H2O2 (1, 2, and 3). The infrared absorption spectrum of gaseous H2O2 has bands at 870, 1370, 2869, and 3417 cm−1. The Raman spectrum of the same sample has bands at 877, 1408, 1435, and 3407 cm−1. All bands correspond to fundamental vibrational wavenumbers and you may assume that:
(a) The 870 and 877 cm−1 bands arise from the same normal mode,
(b) The 3417 and 3407 cm−1 bands arise from the same normal mode.
(i) If H2O2 were linear, how many normal modes of vibration would it have?
(ii) Give the symmetry point group of each of the three proposed conformations of nonlinear H2O2.
(iii) Determine which of the proposed conformations is inconsistent with the spectroscopic data. Explain your reasoning.
Step by Step Answer:

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula





